Mepolizumab for Eosinophil-Associated COPD: Analysis of METREX and METREO
- PMID: 34163157
- PMCID: PMC8215850
- DOI: 10.2147/COPD.S294333
Mepolizumab for Eosinophil-Associated COPD: Analysis of METREX and METREO
Abstract
Background: A pre-specified meta-analysis of individual patient data from the 52-week METREX and METREO trials, which investigated mepolizumab for chronic obstructive pulmonary disease (COPD) in patients with blood eosinophil counts ≥150 cells/µL (screening) or ≥300 cells/µL (prior year) and frequent exacerbations, enables more robust characterization of mepolizumab efficacy in COPD and exploration of the relationship between blood eosinophil count and treatment responses.
Methods: In METREX (117106/NCT02105948) and METREO (117113/NCT02105961), randomized patients received mepolizumab or placebo added to existing inhaled corticosteroid (ICS)-based triple maintenance therapy. The annual rate of moderate/severe exacerbations (primary endpoint) was compared between subcutaneous (SC) mepolizumab 100 mg versus placebo (primary comparison of interest) and all doses (100 mg and 300 mg SC) versus placebo in patients with blood eosinophil counts ≥150 cells/µL at screening or ≥300 cells/µL in the prior year. Secondary/other endpoints included time to first moderate/severe exacerbation, exacerbations leading to emergency department visit/hospitalization and health-related quality of life (HRQoL). A predictive model of the relationship between screening blood eosinophil counts and exacerbation rates included data from all randomized patients.
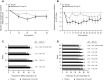
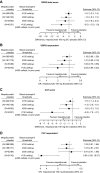
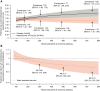
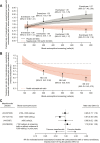
Results: In total, 1510 patients were randomized in METREX and METREO and 1136 patients were included in the pre-specified meta-analysis. From the meta-analysis, mepolizumab 100 mg SC significantly reduced annual moderate/severe exacerbation rates versus placebo by 18% (rate ratio: 0.82; 95% confidence interval: 0.71, 0.95; p=0.006) and delayed time to first moderate/severe exacerbation (hazard ratio: 0.80 [0.68, 0.94]; p=0.006). Mepolizumab 100 mg SC versus placebo numerically reduced exacerbations leading to ED visits/hospitalization and improved HRQoL. A modelling approach demonstrated increasing efficacy for moderate/severe exacerbations with increasing screening blood eosinophil count; this relationship was more pronounced for exacerbations requiring oral corticosteroids (post hoc). The all-doses comparison had similar results.
Conclusion: Mepolizumab reduces exacerbations in patients with eosinophil-associated COPD. Results suggest that blood eosinophil counts (≥150 cells/µL at screening or ≥300 cells/µL in the prior year) allow for identification of patients with COPD who experience exacerbations while treated with maximal ICS-based triple maintenance therapy who are likely to benefit from mepolizumab.
Keywords: COPD; eosinophil; exacerbation; mepolizumab.
© 2021 Pavord et al.
Conflict of interest statement
MB has received honoraria from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Novartis and has received independent/institutional grant support from Pfizer and AstraZeneca. KRC reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, Grifols, Sanofi, Genentech, Kamada, Mereo Biopharma, Regeneron, Roche and Novartis; grants from Baxter, GSK, Vertex, and Amgen; and personal fees from Merck, Takeda. He also holds a Canadian Institute of Health Research-GSK research chair in Respiratory Health Care Delivery at the University Health Network. In the last 5 years, IDP received funding from the NIHR as a Senior Investigator; he has received speaker’s honoraria for speaking at sponsored meetings from AstraZeneca, Boehringer Ingelheim, Aerocrine, Almirall, Menarini, Novartis, Teva, Chiesi and GSK and payments for organizing educational events from AstraZeneca and Teva. He has received honoraria for attending advisory panels with Genentech, Regeneron, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Teva, Merck, Sanofi, Circassia, Chiesi and Knopp. He has received sponsorships to attend international scientific meetings from Boehringer Ingelheim, GSK, AstraZeneca, Teva and Chiesi. He has also received a grant from Chiesi to support a Phase II clinical trial in Oxford. In addition, IDP has a patent Leicester Cough Questionnaire with royalties paid. FCS received grants from the National Institutes of Health, Patient-Centered Outcomes Research Institute, the COPD Foundation, the US Department of Defense, Astellas, AstraZeneca, Boehringer Ingelheim, GSK, Phillips Respironics, PulmonX, PneumRx and Spiration; and personal fees from Circassia, Boehringer Ingelheim, GSK and PneumRx. ESB was an employee of GSK and holds GSK stock/shares during the conduct of the study, his current affiliation is Aeglea BioTherapeutics, Austin, Texas. SWY is an employee of GSK and hold GSK stock/shares. DBR was an employee of GSK and holds GSK stocks/shares during the conduct of the study, his current affiliation is DBR Consulting LLC, Raleigh NC. SSH was an employee of GSK and holds GSK stocks/shares during the conduct of the study. All authors received non-financial support from GSK in the form of editorial support. PP reports personal fees from GlaxoSmith Kline, during the conduct of the study; grants and/or personal fees from AstraZeneca, Chiesi, Novartis, and Sanofi, outside the submitted work. The authors report no other conflicts of interest in this work.
Figures




References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

