Regulated Intramembrane Proteolysis of ACE2: A Potential Mechanism Contributing to COVID-19 Pathogenesis?
- PMID: 34163462
- PMCID: PMC8215698
- DOI: 10.3389/fimmu.2021.612807
Regulated Intramembrane Proteolysis of ACE2: A Potential Mechanism Contributing to COVID-19 Pathogenesis?
Abstract
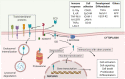
Since being identified as a key receptor for SARS-CoV-2, Angiotensin converting enzyme 2 (ACE2) has been studied as one of the potential targets for the development of preventative and/or treatment options. Tissue expression of ACE2 and the amino acids interacting with the spike protein of SARS-CoV-2 have been mapped. Furthermore, the recombinant soluble extracellular domain of ACE2 is already in phase 2 trials as a treatment for SARS-CoV-2 infection. Most studies have continued to focus on the ACE2 extracellular domain, which is known to play key roles in the renin angiotensin system and in amino acid uptake. However, few also found ACE2 to have an immune-modulatory function and its intracellular tail may be one of the signaling molecules in regulating cellular activation. The implication of its immune-modulatory role in preventing the cytokine-storm, observed in severe COVID-19 disease outcomes requires further investigation. This review focuses on the regulated proteolytic cleavage of ACE2 upon binding to inducer(s), such as the spike protein of SARS-CoV, the potential of cleaved ACE2 intracellular subdomain in regulating cellular function, and the ACE2's immune-modulatory function. This knowledge is critical for targeting ACE2 levels for developing prophylactic treatment or preventative measures in SARS-CoV infections.
Keywords: ACE2; COVID-19; SARS-CoV-2; ectodomain shedding; endodomain cleavage; regulated intramembrane proteolysis.
Copyright © 2021 Gonzalez, Siddik and Su.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Emerging SARS-Cov-2 Variants. CDC (2021). https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/scie....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

