Hyper-Enriched Anti-RSV Immunoglobulins Nasally Administered: A Promising Approach for Respiratory Syncytial Virus Prophylaxis
- PMID: 34163482
- PMCID: PMC8215542
- DOI: 10.3389/fimmu.2021.683902
Hyper-Enriched Anti-RSV Immunoglobulins Nasally Administered: A Promising Approach for Respiratory Syncytial Virus Prophylaxis
Abstract
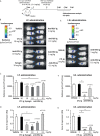
Respiratory syncytial virus (RSV) is a public health concern that causes acute lower respiratory tract infection. So far, no vaccine candidate under development has reached the market and the only licensed product to prevent RSV infection in at-risk infants and young children is a monoclonal antibody (Synagis®). Polyclonal human anti-RSV hyper-immune immunoglobulins (Igs) have also been used but were superseded by Synagis® owing to their low titer and large infused volume. Here we report a new drug class of immunoglobulins, derived from human non hyper-immune plasma that was generated by an innovative bioprocess, called Ig cracking, combining expertises in plasma-derived products and affinity chromatography. By using the RSV fusion protein (F protein) as ligand, the Ig cracking process provided a purified and concentrated product, designated hyper-enriched anti-RSV IgG, composed of at least 15-20% target-specific-antibodies from normal plasma. These anti-RSV Ig displayed a strong in vitro neutralization effect on RSV replication. Moreover, we described a novel prophylactic strategy based on local nasal administration of this unique hyper-enriched anti-RSV IgG solution using a mouse model of infection with bioluminescent RSV. Our results demonstrated that very low doses of hyper-enriched anti-RSV IgG can be administered locally to ensure rapid and efficient inhibition of virus infection. Thus, the general hyper-enriched Ig concept appeared a promising approach and might provide solutions to prevent and treat other infectious diseases.
Importance: Respiratory Syncytial Virus (RSV) is the major cause of acute lower respiratory infections in children, and is also recognized as a cause of morbidity in the elderly. There are still no vaccines and no efficient antiviral therapy against this virus. Here, we described an approach of passive immunization with a new class of hyper-enriched anti-RSV immunoglobulins (Ig) manufactured from human normal plasma. This new class of immunoglobulin plasma derived product is generated by an innovative bioprocess, called Ig cracking, which requires a combination of expertise in both plasma derived products and affinity chromatography. The strong efficacy in a small volume of these hyper-enriched anti-RSV IgG to inhibit the viral infection was demonstrated using a mouse model. This new class of immunoglobulin plasma-derived products could be applied to other pathogens to address specific therapeutic needs in the field of infectious diseases or even pandemics, such as COVID-19.
Keywords: bioprocess; concentration; human plasma; hyper-immune immunoglobulins; nasal administration; neutralization; prophylactic strategy; viral infection.
Copyright © 2021 Jacque, Chottin, Laubreton, Nogre, Ferret, de Marcos, Baptista, Drajac, Mondon, De Romeuf, Rameix-Welti, Eléouët, Chtourou, Riffault, Perret and Descamps.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

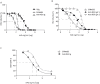



References
-
- Eijkhout HW, van Der Meer JW, Kallenberg CG, Weening RS, van Dissel JT, Sanders LA, et al. . Inter-University Working Party for the Study of Immune: The Effect of Two Different Dosages of Intravenous Immunoglobulin on the Incidence of Recurrent Infections in Patients With Primary Hypogammaglobulinemia. A Randomized, Double-Blind, Multicenter Crossover Trial. Ann Intern Med (2001) 135(3):165–74. 10.7326/0003-4819-135-3-200108070-00008 - DOI - PubMed
-
- Zhu Q, McAuliffe JM, Patel NK, Palmer-Hill FJ, Yang CF, Liang B, et al. . Analysis of Respiratory Syncytial Virus Preclinical and Clinical Variants Resistant to Neutralization by Monoclonal Antibodies Palivizumab and/or Motavizumab. J Infect Dis (2011) 203(5):674–82. 10.1093/infdis/jiq100 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

