Pregnancy Induces an Immunological Memory Characterized by Maternal Immune Alterations Through Specific Genes Methylation
- PMID: 34163485
- PMCID: PMC8215664
- DOI: 10.3389/fimmu.2021.686676
Pregnancy Induces an Immunological Memory Characterized by Maternal Immune Alterations Through Specific Genes Methylation
Abstract
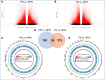
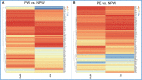
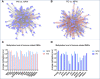
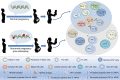
During pregnancy, the maternal immune system undergoes major adaptive modifications that are necessary for the acceptance and protection of the fetus. It has been postulated that these modifications are temporary and limited to the time of pregnancy. Growing evidence suggests that pregnancy has a long-term impact on maternal health, especially among women with pregnancy complications, such as preeclampsia (PE). In addition, the presence of multiple immunological-associated changes in women that remain long after delivery has been reported. To explain these long-term modifications, we hypothesized that pregnancy induces long-term immunological memory with effects on maternal well-being. To test this hypothesis, we evaluated the immunological phenotype of circulating immune cells in women at least 1 year after a normal pregnancy and after pregnancy complicated by PE. Using multiparameter flow cytometry (FCM) and whole-genome bisulfite sequencing (WGBS), we demonstrate that pregnancy has a long-term effect on the maternal immune cell populations and that this effect differs between normal pregnancy and pregnancy complicated by PE; furthermore, these modifications are due to changes in the maternal methylation status of genes that are associated with T cell and NK cell differentiation and function. We propose the existence of an "immunological memory of pregnancy (IMOP)" as an evolutionary advantage for the success of future pregnancies and the proper adaptation to the microchimeric status established during pregnancy. Our findings demonstrate that the type of immune cell populations modified during pregnancy may have an impact on subsequent pregnancy and future maternal health.
Keywords: epigenetic; immune cells; immunological memory; preeclampsia; pregnancy.
Copyright © 2021 Huang, Wang, Zhao, Liu, Chen, Wu, Liu, Ding, Yang, Maxwell, Yin, Mor and Liao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Learning from experience: cellular and molecular bases for improved outcome in subsequent pregnancies.Am J Obstet Gynecol. 2019 Sep;221(3):183-193. doi: 10.1016/j.ajog.2019.02.037. Epub 2019 Feb 22. Am J Obstet Gynecol. 2019. PMID: 30802436 Review.
-
The combination of maternal KIR-B and fetal HLA-C2 is associated with decidua basalis acute atherosis in pregnancies with preeclampsia.J Reprod Immunol. 2018 Sep;129:23-29. doi: 10.1016/j.jri.2018.07.005. Epub 2018 Jul 25. J Reprod Immunol. 2018. PMID: 30055414
-
Analysis of TCR Repertoire and PD-1 Expression in Decidual and Peripheral CD8+ T Cells Reveals Distinct Immune Mechanisms in Miscarriage and Preeclampsia.Front Immunol. 2020 Jun 3;11:1082. doi: 10.3389/fimmu.2020.01082. eCollection 2020. Front Immunol. 2020. PMID: 32582176 Free PMC article.
-
Evaluation of epigenetic age calculators between preeclampsia and normotensive pregnancies in an Australian cohort.Sci Rep. 2022 Jan 31;12(1):1664. doi: 10.1038/s41598-022-05744-4. Sci Rep. 2022. PMID: 35102228 Free PMC article.
-
Preeclampsia and health risks later in life: an immunological link.Semin Immunopathol. 2016 Nov;38(6):699-708. doi: 10.1007/s00281-016-0579-8. Epub 2016 Jun 23. Semin Immunopathol. 2016. PMID: 27339196 Review.
Cited by
-
Role of NLRP7 in Normal and Malignant Trophoblast Cells.Biomedicines. 2022 Jan 24;10(2):252. doi: 10.3390/biomedicines10020252. Biomedicines. 2022. PMID: 35203462 Free PMC article. Review.
-
Setting a stage: Inflammation during preeclampsia and postpartum.Front Physiol. 2023 Feb 23;14:1130116. doi: 10.3389/fphys.2023.1130116. eCollection 2023. Front Physiol. 2023. PMID: 36909242 Free PMC article. Review.
-
Immune checkpoint for pregnancy.Semin Immunopathol. 2025 May 2;47(1):26. doi: 10.1007/s00281-025-01051-y. Semin Immunopathol. 2025. PMID: 40314833 Review.
-
Sexual dimorphism in immunity and longevity among the oldest old.Front Immunol. 2025 Feb 17;16:1525948. doi: 10.3389/fimmu.2025.1525948. eCollection 2025. Front Immunol. 2025. PMID: 40034689 Free PMC article. Review.
-
Emerging Concepts in Innate Lymphoid Cells, Memory, and Reproduction.Front Immunol. 2022 Jun 14;13:824263. doi: 10.3389/fimmu.2022.824263. eCollection 2022. Front Immunol. 2022. PMID: 35774779 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

