Reversing Post-Infectious Epigenetic-Mediated Immune Suppression
- PMID: 34163486
- PMCID: PMC8215363
- DOI: 10.3389/fimmu.2021.688132
Reversing Post-Infectious Epigenetic-Mediated Immune Suppression
Abstract
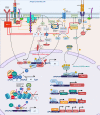
The immune response must balance the pro-inflammatory, cell-mediated cytotoxicity with the anti-inflammatory and wound repair response. Epigenetic mechanisms mediate this balance and limit host immunity from inducing exuberant collateral damage to host tissue after severe and chronic infections. However, following treatment for these infections, including sepsis, pneumonia, hepatitis B, hepatitis C, HIV, tuberculosis (TB) or schistosomiasis, detrimental epigenetic scars persist, and result in long-lasting immune suppression. This is hypothesized to be one of the contributing mechanisms explaining why survivors of infection have increased all-cause mortality and increased rates of unrelated secondary infections. The mechanisms that induce epigenetic-mediated immune suppression have been demonstrated in-vitro and in animal models. Modulation of the AMP-activated protein kinase (AMPK)-mammalian target of rapamycin (mTOR), nuclear factor of activated T cells (NFAT) or nuclear receptor (NR4A) pathways is able to block or reverse the development of detrimental epigenetic scars. Similarly, drugs that directly modify epigenetic enzymes, such as those that inhibit histone deacetylases (HDAC) inhibitors, DNA hypomethylating agents or modifiers of the Nucleosome Remodeling and DNA methylation (NuRD) complex or Polycomb Repressive Complex (PRC) have demonstrated capacity to restore host immunity in the setting of cancer-, LCMV- or murine sepsis-induced epigenetic-mediated immune suppression. A third clinically feasible strategy for reversing detrimental epigenetic scars includes bioengineering approaches to either directly reverse the detrimental epigenetic marks or to modify the epigenetic enzymes or transcription factors that induce detrimental epigenetic scars. Each of these approaches, alone or in combination, have ablated or reversed detrimental epigenetic marks in in-vitro or in animal models; translational studies are now required to evaluate clinical applicability.
Keywords: bioengineering; chronic infections; epigenetics; immune exhaustion; tolerance.
Copyright © 2021 Abhimanyu, Ontiveros, Guerra-Resendez, Nishiguchi, Ladki, Hilton, Schlesinger and DiNardo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

