Towards the stable chelation of radium for biomedical applications with an 18-membered macrocyclic ligand
- PMID: 34163647
- PMCID: PMC8179459
- DOI: 10.1039/d0sc06867e
Towards the stable chelation of radium for biomedical applications with an 18-membered macrocyclic ligand
Abstract
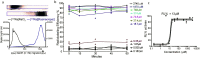
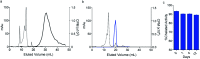
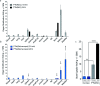
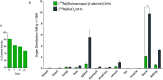
Targeted alpha therapy is an emerging strategy for the treatment of disseminated cancer. [223Ra]RaCl2 is the only clinically approved alpha particle-emitting drug, and it is used to treat castrate-resistant prostate cancer bone metastases, to which [223Ra]Ra2+ localizes. To specifically direct [223Ra]Ra2+ to non-osseous disease sites, chelation and conjugation to a cancer-targeting moiety is necessary. Although previous efforts to stably chelate [223Ra]Ra2+ for this purpose have had limited success, here we report a biologically stable radiocomplex with the 18-membered macrocyclic chelator macropa. Quantitative labeling of macropa with [223Ra]Ra2+ was accomplished within 5 min at room temperature with a radiolabeling efficiency of >95%, representing a significant advancement over conventional chelators such as DOTA and EDTA, which were unable to completely complex [223Ra]Ra2+ under these conditions. [223Ra][Ra(macropa)] was highly stable in human serum and exhibited dramatically reduced bone and spleen uptake in mice in comparison to bone-targeted [223Ra]RaCl2, signifying that [223Ra][Ra(macropa)] remains intact in vivo. Upon conjugation of macropa to a single amino acid β-alanine as well as to the prostate-specific membrane antigen-targeting peptide DUPA, both constructs retained high affinity for 223Ra, complexing >95% of Ra2+ in solution. Furthermore, [223Ra][Ra(macropa-β-alanine)] was rapidly cleared from mice and showed low 223Ra bone absorption, indicating that this conjugate is stable under biological conditions. Unexpectedly, this stability was lost upon conjugation of macropa to DUPA, which suggests a role of targeting vectors in complex stability in vivo for this system. Nonetheless, our successful demonstration of efficient radiolabeling of the β-alanine conjugate with 223Ra and its subsequent stability in vivo establishes for the first time the possibility of delivering [223Ra]Ra2+ to metastases outside of the bone using functionalized chelators, marking a significant expansion of the therapeutic utility of this radiometal in the clinic.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Wilson and Thiele hold intellectual property rights on macropa; Abou and Thorek have filed provisional patent protection for radium-related production and utilization through WUSTL. Justin Wilson holds equity in Noria Therapeutics, Inc., which has licensed this technology.
Figures


References
-
- Kluetz P. G. Pierce W. Maher V. E. Zhang H. Tang S. Song P. Liu Q. Haber M. T. Leutzinger E. E. Al-Hakim A. Chen W. Palmby T. Alebachew E. Sridhara R. Ibrahim A. Justice R. Pazdur R. Clin. Cancer Res. 2014;20:9–14. - PubMed
-
- Parker C. Nilsson D Heinrich S. Helle S. I. O'Sullivan J. M. Fosså S. D. Chodacki A. Wiechno P. Logue J. Seke M. Widmark A. Johannessen D. C. Hoskin P. Bottomley D. James N. D. Solberg A. Syndikus I. Kliment J. Wedel S. Boehmer S. Dall'Oglio M. Franzén L. Coleman R. Vogelzang N. J. O'Bryan-Tear C. G. Staudacher K. Garcia-Vargas J. Shan M. Bruland S. Sartor O. N. Engl. J. Med. 2013;369:213–223. - PubMed
-
- Sartor O. Coleman R. Nilsson S. Heinrich D. Helle S. I. O'Sullivan J. M. Fosså S. D. Chodacki A. Wiechno P. Logue J. Widmark A. Johannessen D. C. Hoskin P. James N. D. Solberg A. Syndikus I. Vogelzang N. J. O'Bryan-Tear C. G. Shan M. Bruland Ø. S. Parker C. Lancet Oncol. 2014;15:738–746. - PubMed
-
- Henriksen G. Fisher D. R. Roeske J. C. Bruland Ø. S. Larsen R. H. J. Nucl. Med. 2003;44:252–259. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

