Hexafluoroisopropanol: the magical solvent for Pd-catalyzed C-H activation
- PMID: 34163654
- PMCID: PMC8179444
- DOI: 10.1039/d0sc06937j
Hexafluoroisopropanol: the magical solvent for Pd-catalyzed C-H activation
Abstract
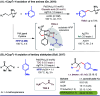
Among numerous solvents available for chemical transformations, 1,1,1,3,3,3-hexafluoro-2-propanol (popularly known as HFIP) has attracted enough attention of the scientific community in recent years. Several unique features of HFIP compared to its non-fluoro analogue isopropanol have helped this solvent to make a difference in various subdomains of organic chemistry. One such area is transition metal-catalyzed C-H bond functionalization reactions. While, on one side, HFIP is emerging as a green and sustainable deep eutectic solvent (DES), on the other side, a major proportion of Pd-catalyzed C-H functionalization is heavily relying on this solvent. In particular, for distal aromatic C-H functionalizations, the exceptional impact of HFIP to elevate the yield and selectivity has made this solvent irreplaceable. Recent research studies have also highlighted the H-bond-donating ability of HFIP to enhance the chiral induction in Pd-catalyzed atroposelective C-H activation. This perspective aims to portray different shades of HFIP as a magical solvent in Pd-catalyzed C-H functionalization reactions.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


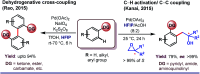



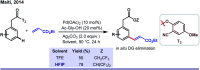
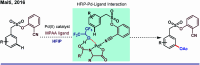
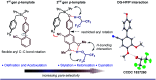

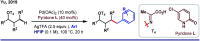
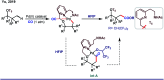
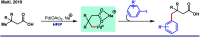








References
-
-
This market value of HFIP is taken from Synquest Laboratories. The market price for HFIP according to European currency is £99 per kg (from Fluorochem). While handling HFIP safety precautions as per standard MSDS has to be thoroughly maintained
-
-
- An X.-D. Xiao J. Chem. Rec. 2019;19:1–21. doi: 10.1002/tcr.201980101. - DOI
-
- Ravikumar K. S. Zhang Y. M. Begue J.-P. Bonnet-Delpon D. Eur. J. Org. Chem. 1998:2937–2940. doi: 10.1002/(SICI)1099-0690(199812)1998:12<2937::AID-EJOC2937>3.0.CO;2-D. - DOI
- Neimann K. Neumann R. Org. Lett. 2000;2:2861–2863. doi: 10.1021/ol006287m. - DOI - PubMed
- Hollóczki O. Berkessel A. Mars J. Mezger M. Wiebe A. Waldvogel S. R. Kirchner B. ACS Catal. 2017;7:1846–1852. doi: 10.1021/acscatal.6b03090. - DOI
- Berkessel A. Adrio J. A. J. Am. Chem. Soc. 2006;128:13412–13420. doi: 10.1021/ja0620181. - DOI - PubMed
- Berkessel A. Adrio J. A. Hüttenhain D. Neudörfl J. M. J. Am. Chem. Soc. 2006;128:8421–8426. doi: 10.1021/ja0545463. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

