Access to P-stereogenic compounds via desymmetrizing enantioselective bromination
- PMID: 34163723
- PMCID: PMC8179578
- DOI: 10.1039/d0sc07008d
Access to P-stereogenic compounds via desymmetrizing enantioselective bromination
Abstract
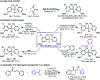
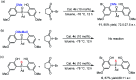
A novel and efficient desymmetrizing asymmetric ortho-selective mono-bromination of bisphenol phosphine oxides under chiral squaramide catalysis was reported. Using this asymmetric ortho-bromination strategy, a wide range of chiral bisphenol phosphine oxides and bisphenol phosphinates were obtained with good to excellent yields (up to 92%) and enantioselectivities (up to 98.5 : 1.5 e.r.). The reaction could be scaled up, and the synthetic utility of the desired P-stereogenic compounds was proved by transformations and application in an asymmetric reaction.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources

