Coupling molecular rigidity and flexibility on fused backbones for NIR-II photothermal conversion
- PMID: 34163755
- PMCID: PMC8179590
- DOI: 10.1039/d1sc00060h
Coupling molecular rigidity and flexibility on fused backbones for NIR-II photothermal conversion
Abstract
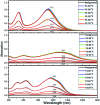
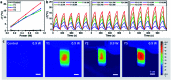
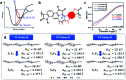
Great attention is being increasingly paid to photothermal conversion in the near-infrared (NIR)-II window (1000-1350 nm), where deeper tissue penetration is favored. To date, only a limited number of organic photothermal polymers and relevant theory have been exploited to direct the molecular design of polymers with highly efficient photothermal conversion, specifically in the NIR-II window. This work proposes a fused backbone structure locked via an intramolecular hydrogen bonding interaction and double bond, which favors molecular planarity and rigidity in the ground state and molecular flexibility in the excited state. Following this proposal, a particular class of NIR-II photothermal polymers are prepared. Their remarkable photothermal conversion efficiency is in good agreement with our strategy of coupling polymeric rigidity and flexibility, which accounts for the improved light absorption on going from the ground state to the excited state and nonradiative emission on going from the excited state to the ground state. It is envisioned that such a concept of coupling polymeric rigidity and flexibility will offer great inspiration for developing NIR-II photothermal polymers with the use of other chromophores.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

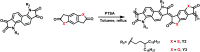

Similar articles
-
A Borondifluoride-Complex-Based Photothermal Agent with an 80 % Photothermal Conversion Efficiency for Photothermal Therapy in the NIR-II Window.Angew Chem Int Ed Engl. 2021 Oct 4;60(41):22376-22384. doi: 10.1002/anie.202107836. Epub 2021 Aug 20. Angew Chem Int Ed Engl. 2021. PMID: 34289230
-
Quinoid Conjugated Polymer Nanoparticles with NIR-II Absorption Peak Toward Efficient Photothermal Therapy.Chemistry. 2023 Mar 7;29(14):e202202930. doi: 10.1002/chem.202202930. Epub 2023 Feb 8. Chemistry. 2023. PMID: 36484147
-
A thieno-isoindigo derivative-based conjugated polymer nanoparticle for photothermal therapy in the NIR-II bio-window.Nanoscale. 2020 Oct 8;12(38):19665-19672. doi: 10.1039/d0nr03771k. Nanoscale. 2020. PMID: 32966502
-
Recent Progress on NIR-II Photothermal Therapy.Front Chem. 2021 Jul 29;9:728066. doi: 10.3389/fchem.2021.728066. eCollection 2021. Front Chem. 2021. PMID: 34395388 Free PMC article. Review.
-
Near-infrared-II activated inorganic photothermal nanomedicines.Biomaterials. 2021 Feb;269:120459. doi: 10.1016/j.biomaterials.2020.120459. Epub 2020 Oct 24. Biomaterials. 2021. PMID: 33139071 Review.
Cited by
-
Gd2O3/b-TiO2 composite nanoprobes with ultra-high photoconversion efficiency for MR image-guided NIR-II photothermal therapy.Exploration (Beijing). 2022 Jun 13;2(6):20220014. doi: 10.1002/EXP.20220014. eCollection 2022 Dec. Exploration (Beijing). 2022. PMID: 37324803 Free PMC article.
-
High-Performance Semiconducting Carbon Nanotube Transistors Using Naphthalene Diimide-Based Polymers with Biaxially Extended Conjugated Side Chains.ACS Appl Mater Interfaces. 2024 Aug 28;16(34):45275-45288. doi: 10.1021/acsami.4c08981. Epub 2024 Aug 13. ACS Appl Mater Interfaces. 2024. PMID: 39137092 Free PMC article.
-
Subcutaneous power supply by NIR-II light.Nat Commun. 2022 Nov 3;13(1):6596. doi: 10.1038/s41467-022-34047-5. Nat Commun. 2022. PMID: 36329024 Free PMC article.
References
-
- Yang Y. He P. Wang Y. Bai H. Wang S. Xu J.-F. Zhang X. Angew. Chem., Int. Ed. 2017;56:16239–16242. - PubMed
-
- He Y. Cao Y. Wang Y. Asian J. Org. Chem. 2018;7:2201–2212.
-
- Jiang Y. Li J. Zhen X. Xie C. Pu K. Adv. Mater. 2018;30:1705980. - PubMed
-
- Li B. Lu L. Zhao M. Lei Z. Zhang F. Angew. Chem., Int. Ed. 2018;57:7483–7487. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

