Charge stabilization via electron exchange: excited charge separation in symmetric, central triphenylamine derived, dimethylaminophenyl-tetracyanobutadiene donor-acceptor conjugates
- PMID: 34163878
- PMCID: PMC8179009
- DOI: 10.1039/d0sc04648e
Charge stabilization via electron exchange: excited charge separation in symmetric, central triphenylamine derived, dimethylaminophenyl-tetracyanobutadiene donor-acceptor conjugates
Abstract
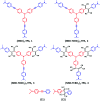
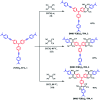
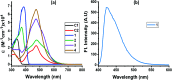
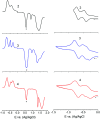
Photoinduced charge separation in donor-acceptor conjugates plays a pivotal role in technology breakthroughs, especially in the areas of efficient conversion of solar energy into electrical energy and fuels. Extending the lifetime of the charge separated species is a necessity for their practical utilization, and this is often achieved by following the mechanism of natural photosynthesis where the process of electron/hole migration occurs distantly separating the radical ion pairs. Here, we hypothesize and demonstrate a new mechanism to stabilize the charge separated states via the process of electron exchange among the different acceptor entities in multimodular donor-acceptor conjugates. For this, star-shaped, central triphenylamine derived, dimethylamine-tetracyanobutadiene conjugates have been newly designed and characterized. Electron exchange was witnessed upon electroreduction in conjugates having multiple numbers of electron acceptors. Using ultrafast spectroscopy, the occurrence of excited state charge separation, and the effect of electron exchange in prolonging the lifetime of charge separated states in the conjugates having multiple acceptors have been successfully demonstrated. This work constitutes the first example of stabilizing charge-separated states via the process of electron exchange.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- Bottari G. and Torres T., Organic Nanomaterials: Synthesis, Characterization, and Device Applications, ed. T. Torres and G. Bottari, John Wiley & Sons, Inc., Hoboken, NJ, 2013
LinkOut - more resources
Full Text Sources

