Regiodivergent synthesis of pyrazino-indolines vs. triazocines via α-imino carbenes addition to imidazolidines
- PMID: 34163911
- PMCID: PMC8179195
- DOI: 10.1039/d0sc05725h
Regiodivergent synthesis of pyrazino-indolines vs. triazocines via α-imino carbenes addition to imidazolidines
Abstract
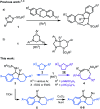
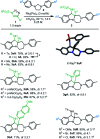
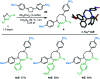
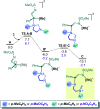
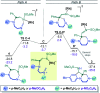
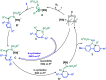
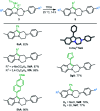
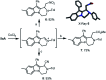
Hexahydropyrazinoindoles were prepared in a single step from N-sulfonyl triazoles and imidazolidines. Under dirhodium catalysis, α-imino carbenes were generated and formed nitrogen ylide intermediates that, after subsequent aminal opening, afforded the pyrazinoindoles predominantly via formal [1,2]-Stevens and tandem Friedel-Crafts cyclizations. Of mechanistic importance, a regiodivergent reactivity was engineered through the use of a specific unsymmetrically substituted imidazolidine that promoted the exclusive formation of 8-membered ring 1,3,6-triazocines. Based on DFT calculations, an original Curtin-Hammett-like situation was demonstrated for the mechanism. Further derivatizations led to functionalized tetrahydropyrazinoindoles in high yields.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

References
-
- Meldal M. Tornøe C. W. Chem. Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. - DOI - PubMed
- Hein J. E. Fokin V. V. Chem. Soc. Rev. 2010;39:1302–1315. doi: 10.1039/B904091A. - DOI - PMC - PubMed
- Schulze B. Schubert U. S. Chem. Soc. Rev. 2014;43:2522–2571. doi: 10.1039/C3CS60386E. - DOI - PubMed
- Tiwari V. K. Mishra B. B. Mishra K. B. Mishra N. Singh A. S. Chen X. Chem. Rev. 2016;116:3086–3240. doi: 10.1021/acs.chemrev.5b00408. - DOI - PubMed
- Haugland M. M. Borsley S. Cairns-Gibson D. F. Elmi A. Cockroft S. L. ACS Nano. 2019;13:4101–4110. doi: 10.1021/acsnano.8b08691. - DOI - PubMed
-
- Kolb H. C. Finn M. G. Sharpless K. B. Angew. Chem., Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. - DOI - PubMed
- Lewis W. G. Green L. G. Grynszpan F. Radić Z. Carlier P. R. Taylor P. Finn M. G. Sharpless K. B. Angew. Chem., Int. Ed. 2002;41:1053–1057. doi: 10.1002/1521-3773(20020315)41:6<1053::AID-ANIE1053>3.0.CO;2-4. - DOI - PubMed
- Amblard F. Cho J. H. Schinazi R. F. Chem. Rev. 2009;109:4207–4220. doi: 10.1021/cr9001462. - DOI - PMC - PubMed
- Le Droumaguet C. Wang C. Wang Q. Chem. Soc. Rev. 2010;39:1233–1239. doi: 10.1039/B901975H. - DOI - PubMed
- Thirumurugan P. Matosiuk D. Jozwiak K. Chem. Rev. 2013;113:4905–4979. doi: 10.1021/cr200409f. - DOI - PubMed
-
- Grünanger P. Finzi P. V. Scotti C. Chem. Ber. 1965;98:623–628. doi: 10.1002/cber.19650980241. - DOI
- Hermes M. E. Marsh F. D. J. Am. Chem. Soc. 1967;89:4760–4764. doi: 10.1021/ja00994a034. - DOI
- Harmon R. E. Stanley F. Gupta S. K. Johnson J. J. Org. Chem. 1970;35:3444–3448. doi: 10.1021/jo00835a057. - DOI
- Harmon R. E. Earl R. A. Gupta S. K. J. Chem. Soc. D. 1971:296–297. doi: 10.1039/C29710000296. - DOI
-
- Chattopadhyay B. Gevorgyan V. Angew. Chem., Int. Ed. 2012;51:862–872. doi: 10.1002/anie.201104807. - DOI - PMC - PubMed
- Gulevich A. V. Gevorgyan V. Angew. Chem., Int. Ed. 2013;52:1371–1373. doi: 10.1002/anie.201209338. - DOI - PMC - PubMed
- Davies H. M. Alford J. S. Chem. Soc. Rev. 2014;43:5151–5162. doi: 10.1039/C4CS00072B. - DOI - PubMed
- Anbarasan P. Yadagiri D. Rajasekar S. Synthesis. 2014;46:3004–3023. doi: 10.1055/s-0034-1379303. - DOI
- Wang Y. Lei X. Tang Y. Synlett. 2015;26:2051–2059. doi: 10.1055/s-0034-1380444. - DOI
- Jiang Y. Sun R. Tang X.-Y. Shi M. Chem.–Eur. J. 2016;22:17910–17924. doi: 10.1002/chem.201601703. - DOI - PubMed
-
- Chuprakov S. Hwang F. W. Gevorgyan V. Angew. Chem., Int. Ed. 2007;46:4757–4759. doi: 10.1002/anie.200700804. - DOI - PMC - PubMed
- Chuprakov S. Kwok S. W. Zhang L. Lercher L. Fokin V. V. J. Am. Chem. Soc. 2009;131:18034–18035. doi: 10.1021/ja908075u. - DOI - PMC - PubMed
- Chuprakov S. Malik J. A. Zibinsky M. Fokin V. V. J. Am. Chem. Soc. 2011;133:10352–10355. doi: 10.1021/ja202969z. - DOI - PMC - PubMed
- Zibinsky M. Fokin V. V. Org. Lett. 2011;13:4870–4872. doi: 10.1021/ol201949h. - DOI - PMC - PubMed
- Yadagiri D. Anbarasan P. Chem.–Eur. J. 2013;19:15115–15119. doi: 10.1002/chem.201302653. - DOI - PubMed
- Schultz E. E. Sarpong R. J. Am. Chem. Soc. 2013;135:4696–4699. doi: 10.1021/ja401380d. - DOI - PubMed
- Miura T. Tanaka T. Matsumoto K. Murakami M. Chem.–Eur. J. 2014;20:16078–16082. doi: 10.1002/chem.201405357. - DOI - PubMed
- Miura T. Nakamuro T. Liang C.-J. Murakami M. J. Am. Chem. Soc. 2014;136:15905–15908. doi: 10.1021/ja5096045. - DOI - PubMed
- Yadagiri D. Anbarasan P. Org. Lett. 2014;16:2510–2513. doi: 10.1021/ol500874p. - DOI - PubMed
- Medina F. Besnard C. Lacour J. Org. Lett. 2014;16:3232–3235. doi: 10.1021/ol5012532. - DOI - PubMed
- Lindsay V. N. G. Viart H. M. F. Sarpong R. J. Am. Chem. Soc. 2015;137:8368–8371. doi: 10.1021/jacs.5b04295. - DOI - PubMed
- Kubiak R. W. Mighion J. D. Wilkerson-Hill S. M. Alford J. S. Yoshidomi T. Davies H. M. L. Org. Lett. 2016;18:3118–3121. doi: 10.1021/acs.orglett.6b01298. - DOI - PMC - PubMed
- Guarnieri-Ibáñez A. Medina F. Besnard C. Kidd S. L. Spring D. R. Lacour J. Chem. Sci. 2017;8:5713–5720. doi: 10.1039/C7SC00964J. - DOI - PMC - PubMed
- Miura T. Zhao Q. Murakami M. Angew. Chem., Int. Ed. 2017;56:16645–16649. doi: 10.1002/anie.201709384. - DOI - PubMed
- Ma X. Xie X. Liu L. Xia R. Li T. Wang H. Chem. Commun. 2018;54:1595–1598. doi: 10.1039/C7CC08438B. - DOI - PubMed
- Liu Z. Du Q. Zhai H. Li Y. Org. Lett. 2018;20:7514–7517. doi: 10.1021/acs.orglett.8b03275. - DOI - PubMed
- Garlets Z. J. Davies H. M. L. Org. Lett. 2018;20:2168–2171. doi: 10.1021/acs.orglett.8b00427. - DOI - PMC - PubMed
- Jia R. Meng J. Leng J. Yu X. Deng W. P. Chem.–Asian J. 2018;13:2360–2364. doi: 10.1002/asia.201800057. - DOI - PubMed
- Yadagiri D. Chaitanya M. Reddy A. C. S. Anbarasan P. Org. Lett. 2018;20:3762–3765. doi: 10.1021/acs.orglett.8b01338. - DOI - PubMed
- Xu Z.-F. Shan L. Zhang W. Cen M. Li C.-Y. Org. Chem. Front. 2019;6:1391–1396. doi: 10.1039/C9QO00126C. - DOI
- De P. B. Atta S. Pradhan S. Banerjee S. Shah T. A. Punniyamurthy T. J. Org. Chem. 2020;85:4785–4794. doi: 10.1021/acs.joc.0c00010. - DOI - PubMed
- Reddy A. C. S. Ramachandran K. Reddy P. M. Anbarasan P. Chem. Commun. 2020;56:5649–5652. doi: 10.1039/D0CC00016G. - DOI - PubMed
- Dequina H. J. Eshon J. Raskopf W. T. Fernández I. Schomaker J. M. Org. Lett. 2020;22:3637–3641. doi: 10.1021/acs.orglett.0c01124. - DOI - PMC - PubMed
- Miura T. Nakamuro T. Ishihara Y. Nagata Y. Murakami M. Angew. Chem., Int. Ed. 2020;59:20475–20479. doi: 10.1002/anie.202009781. - DOI - PubMed
LinkOut - more resources
Full Text Sources

