Tri-insertion with dearomatization of terminal arylalkynes using a carborane based frustrated Lewis pair template
- PMID: 34163934
- PMCID: PMC8179331
- DOI: 10.1039/d0sc05755j
Tri-insertion with dearomatization of terminal arylalkynes using a carborane based frustrated Lewis pair template
Abstract
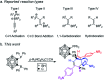
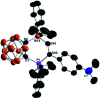
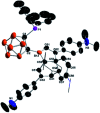
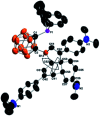
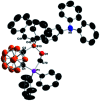
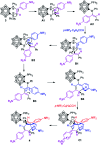
Intramolecular vicinal Frustrated Lewis Pairs (FLPs) have played a significant role in the activation of small molecules, and their stabilities and reactivities are found to strongly depend on the nature of the bridging units. This work reports a new carborane based FLP, 1-PPh2-2-BPh2-1,2-C2B10H10 (2), which reacts with an equimolar amount of p-R2NC6H4C[triple bond, length as m-dash]CH (R = Me, Et, Ph) at room temperature to give C[triple bond, length as m-dash]C triple bond addition products 1,2-[PPh2C(R2NC6H4)[double bond, length as m-dash]CHBPh2]-1,2-C2B10H10 (3) in high yields. Compounds 3 react further with two equiv. of p-R2NC6H4C[triple bond, length as m-dash]CH (R = Me, Et) at 60-70 °C to give unprecedented stereoselective tri-insertion products, 3,3a,6,6a-tetrahydronaphtho[1,8a-b]borole tricycles (4), in which one of the aryl rings from arylacetylene moieties has been dearomatized with the formation of four stereocenters including one quaternary carbon center. It is noted that the phosphine unit functions as a catalyst during the reactions. After trapping and structural characterization of a key intermediate, a reaction mechanism is proposed, involving sequential alkyne insertion and 1,2-boryl migration.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
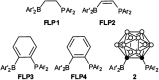

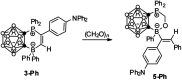
Similar articles
-
Amino group combined P/Ge and P/Sn Lewis pairs: synthesis and dipolar addition reactions to alkyne and aldehyde molecules.Dalton Trans. 2016 Apr 14;45(14):6259-68. doi: 10.1039/c5dt03873a. Epub 2015 Dec 14. Dalton Trans. 2016. PMID: 26658532
-
C-C coupling reactions in the coordination sphere of rhodium(I) and rhodium(III): New routes for the di- and trimerization of terminal alkynes.Dalton Trans. 2005 Apr 21;(8):1468-81. doi: 10.1039/b415541f. Epub 2005 Mar 10. Dalton Trans. 2005. PMID: 15824785
-
A P-H functionalized Al/P-based frustrated Lewis pair - hydrophosphination of nitriles, ring opening with cyclopropenones and evidence of P[double bond, length as m-dash]C double bond formation.Dalton Trans. 2018 Jun 25;47(25):8402-8417. doi: 10.1039/c8dt01836g. Dalton Trans. 2018. PMID: 29893387
-
Reactions of α-diimine-aluminum complexes with sodium alkynides: versatile structures of aluminum σ-alkynide complexes.Dalton Trans. 2015 Aug 14;44(30):13671-80. doi: 10.1039/c5dt01693b. Dalton Trans. 2015. PMID: 26147659
-
Equilibria and mesomerism/valence tautomerism of group 4 metallocene complexes.Chem Soc Rev. 2020 Apr 7;49(7):2119-2139. doi: 10.1039/c9cs00637k. Chem Soc Rev. 2020. PMID: 32186300 Review.
Cited by
-
Exploring the Reactivity of B-Connected Carboranylphosphines in Frustrated Lewis Pair Chemistry: A New Frame for a Classic System.Chemistry. 2022 Jun 15;28(34):e202200531. doi: 10.1002/chem.202200531. Epub 2022 May 12. Chemistry. 2022. PMID: 35472172 Free PMC article.
References
-
-
For recent reviews, see:
- Stephan D. W. Acc. Chem. Res. 2015;48:306–316. doi: 10.1021/ar500375j. - DOI - PubMed
- Stephan D. W. Erker G. Angew. Chem., Int. Ed. 2015;54:6400–6441. doi: 10.1002/anie.201409800. - DOI - PubMed
- Stephan D. W. J. Am. Chem. Soc. 2015;137:10018–10032. doi: 10.1021/jacs.5b06794. - DOI - PubMed
- Cardenas A. J. P. Hasegawa Y. Kehr G. Warren T. H. Erker G. Coord. Chem. Rev. 2016;306:468–482. doi: 10.1016/j.ccr.2015.01.006. - DOI
- Stephan D. W. Science. 2016;354:aaf7229. doi: 10.1126/science.aaf7229. - DOI - PubMed
- Paradies J. Coord. Chem. Rev. 2019;380:170–183. doi: 10.1016/j.ccr.2018.09.014. - DOI
- Jupp A. R. Stephan D. W. Trends Chem. 2019;1:25–48.
-
-
- McCahill J. S. J. Welch G. C. Stephan D. W. Angew. Chem., Int. Ed. 2007;46:4968–4971. doi: 10.1002/anie.200701215. - DOI - PubMed
- Fukazawa A. Yamada H. Yamaguchi S. Angew. Chem., Int. Ed. 2008;47:5582–5585. doi: 10.1002/anie.200801834. - DOI - PubMed
- Dureen M. A. Stephan D. W. J. Am. Chem. Soc. 2009;131:8396–8397. doi: 10.1021/ja903650w. - DOI - PubMed
- Tamke S. Qu Z.-W. Sitte N. A. Flörke U. Grimme S. Paradies J. Angew. Chem., Int. Ed. 2016;55:4336–4339. doi: 10.1002/anie.201511921. - DOI - PubMed
-
- Mömming C. M. Frömel S. Kehr G. Fröhlich R. Grimme S. Erker G. J. Am. Chem. Soc. 2009;131:12l280–12289. doi: 10.1021/ja903511s. - DOI - PubMed
- Rosorius C. Kehr G. Fröhlich R. Grimme S. Erker G. Organometallics. 2011;30:4211–4219. doi: 10.1021/om200569k. - DOI
- Jie X. Daniliuc C. G. Knitsch R. Hansen M. R. Eckert H. Ehlert S. Grimme S. Kehr G. Erker G. Angew. Chem., Int. Ed. 2019;58:882–886. doi: 10.1002/anie.201811873. - DOI - PubMed
- Dong S. Daniliuc C. G. Kehr G. Erker G. Chem.–Eur. J. 2020;26:745–753. doi: 10.1002/chem.201904919. - DOI - PubMed
-
- Mömming C. M. Kehr G. Wibbeling B. Fröhlich R. Erker G. Dalton Trans. 2010;39:7556–7564. doi: 10.1039/C0DT00015A. - DOI - PubMed
- Ekkert O. Kehr G. Daniliuc C. G. Fröhlich R. Wibbeling B. Petersen J. L. Erker G. Z. Anorg. Allg. Chem. 2013;639:2455–2462. doi: 10.1002/zaac.201300421. - DOI
- Uhl W. Appelt C. Organometallics. 2013;32:5008–5014. doi: 10.1021/om400620h. - DOI
LinkOut - more resources
Full Text Sources

