Small peptide diversification through photoredox-catalyzed oxidative C-terminal modification
- PMID: 34164012
- PMCID: PMC8179259
- DOI: 10.1039/d0sc06180h
Small peptide diversification through photoredox-catalyzed oxidative C-terminal modification
Abstract
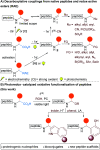
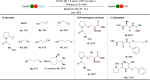
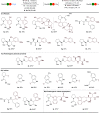
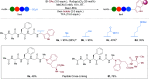
A photoredox-catalyzed oxidative decarboxylative coupling of small peptides is reported, giving access to a variety of N,O-acetals. They were used as intermediates for the addition of phenols and indoles, leading to novel peptide scaffolds and bioconjugates. Amino acids with nucleophilic side chains, such as serine, threonine, tyrosine and tryptophan, could also be used as partners to access tri- and tetrapeptide derivatives with non-natural cross-linking.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Fosgerau K. Hoffmann T. Drug Discov. Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. - DOI - PubMed
- Dang T. Süssmuth R. D. Acc. Chem. Res. 2017;50:1566–1576. doi: 10.1021/acs.accounts.7b00159. - DOI - PubMed
- Hamley I. W. Chem. Rev. 2017;117:14015–14041. doi: 10.1021/acs.chemrev.7b00522. - DOI - PubMed
- Henninot A. Collins J. C. Nuss J. M. J. Med. Chem. 2018;61:1382–1414. doi: 10.1021/acs.jmedchem.7b00318. - DOI - PubMed
-
- Ueda T. Biochim. Biophys. Acta Protein Proteonomics. 2014;1844:2053–2057. doi: 10.1016/j.bbapap.2014.06.008. - DOI - PubMed
- Drake P. M. Rabuka D. Curr. Opin. Chem. Biol. 2015;28:174–180. doi: 10.1016/j.cbpa.2015.08.005. - DOI - PubMed
- Cohen D. T. Zhang C. Fadzen C. M. Mijalis A. J. Hie L. Johnson K. D. Shriver Z. Plante O. Miller S. J. Buchwald S. L. Pentelute B. L. Nat. Chem. 2019;11:78–85. doi: 10.1038/s41557-018-0154-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

