Does liquid-liquid phase separation drive peptide folding?
- PMID: 34164013
- PMCID: PMC8179267
- DOI: 10.1039/d0sc04993j
Does liquid-liquid phase separation drive peptide folding?
Abstract
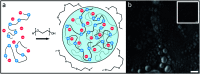
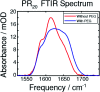
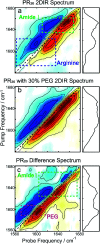
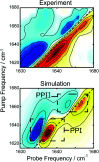
Proline-arginine (PR) dipeptide repeats have been shown to undergo liquid-liquid phase separation and are an example of a growing number of intrinsically disordered proteins that can assemble into membraneless organelles. These structures have been posited as nucleation sites for pathogenic protein aggregation. As such, a better understanding of the effects that the increased local concentration and volumetric crowding within droplets have on peptide secondary structure is necessary. Herein we use Fourier transform infrared (FTIR) and two-dimensional infrared (2DIR) spectroscopy to show that formation of droplets by PR20 accompanies changes in the amide-I spectra consistent with folding into poly-proline helical structures.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Liquid-Liquid Phase Separation in Crowded Environments.Int J Mol Sci. 2020 Aug 17;21(16):5908. doi: 10.3390/ijms21165908. Int J Mol Sci. 2020. PMID: 32824618 Free PMC article. Review.
-
Intrinsically disordered proteins in crowded milieu: when chaos prevails within the cellular gumbo.Cell Mol Life Sci. 2018 Nov;75(21):3907-3929. doi: 10.1007/s00018-018-2894-9. Epub 2018 Jul 31. Cell Mol Life Sci. 2018. PMID: 30066087 Free PMC article. Review.
-
Protein misfolding and amyloid nucleation through liquid-liquid phase separation.Chem Soc Rev. 2024 May 20;53(10):4976-5013. doi: 10.1039/d3cs01065a. Chem Soc Rev. 2024. PMID: 38597222 Review.
-
TAR DNA-binding protein 43 (TDP-43) liquid-liquid phase separation is mediated by just a few aromatic residues.J Biol Chem. 2018 Apr 20;293(16):6090-6098. doi: 10.1074/jbc.AC117.001037. Epub 2018 Mar 6. J Biol Chem. 2018. PMID: 29511089 Free PMC article.
-
Fourier transform infrared spectroscopy of intrinsically disordered proteins: measurement procedures and data analyses.Methods Mol Biol. 2012;895:229-44. doi: 10.1007/978-1-61779-927-3_16. Methods Mol Biol. 2012. PMID: 22760323
Cited by
-
COCOMO2: A Coarse-Grained Model for Interacting Folded and Disordered Proteins.J Chem Theory Comput. 2025 Feb 25;21(4):2095-2107. doi: 10.1021/acs.jctc.4c01460. Epub 2025 Feb 5. J Chem Theory Comput. 2025. PMID: 39908323 Free PMC article.
-
Biophysical studies of phase separation integrating experimental and computational methods.Curr Opin Struct Biol. 2021 Oct;70:78-86. doi: 10.1016/j.sbi.2021.04.004. Epub 2021 Jun 15. Curr Opin Struct Biol. 2021. PMID: 34144468 Free PMC article. Review.
-
Toward Accurate Simulation of Coupling between Protein Secondary Structure and Phase Separation.J Am Chem Soc. 2024 Jan 10;146(1):342-357. doi: 10.1021/jacs.3c09195. Epub 2023 Dec 19. J Am Chem Soc. 2024. PMID: 38112495 Free PMC article.
-
Liquid-Liquid Phase Separation of the Intrinsically Disordered Domain of the Fused in Sarcoma Protein Results in Substantial Slowing of Hydration Dynamics.J Phys Chem Lett. 2023 Dec 14;14(49):11224-11234. doi: 10.1021/acs.jpclett.3c02790. Epub 2023 Dec 6. J Phys Chem Lett. 2023. PMID: 38056002 Free PMC article.
-
The Proteome Folding Problem and Cellular Proteostasis.J Mol Biol. 2021 Oct 1;433(20):167197. doi: 10.1016/j.jmb.2021.167197. Epub 2021 Aug 13. J Mol Biol. 2021. PMID: 34391802 Free PMC article. Review.
References
-
- De Kruif C. G. Weinbreck F. De Vries R. Curr. Opin. Colloid Interface Sci. 2004;9:340–349. doi: 10.1016/j.cocis.2004.09.006. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

