Synthesis and characterization of carbene derivatives of Th@ C 3v(8)-C82 and U@ C 2v(9)-C82: exceptional chemical properties induced by strong actinide-carbon cage interaction
- PMID: 34164015
- PMCID: PMC8179337
- DOI: 10.1039/d0sc06111e
Synthesis and characterization of carbene derivatives of Th@ C 3v(8)-C82 and U@ C 2v(9)-C82: exceptional chemical properties induced by strong actinide-carbon cage interaction
Abstract
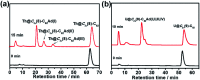
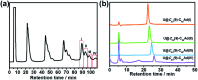
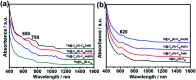
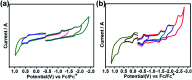
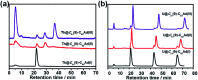
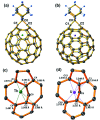
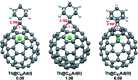
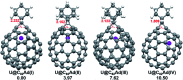
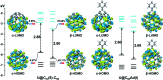
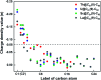
Chemical functionalization of endohedral metallofullerenes (EMFs) is essential for the application of these novel carbon materials. Actinide EMFs, a new EMF family member, have presented unique molecular and electronic structures but their chemical properties remain unexplored. Here, for the first time, we report the chemical functionalization of actinide EMFs, in which the photochemical reaction of Th@C 3v(8)-C82 and U@C 2v(9)-C82 with 2-adamantane-2,3'-[3H]-diazirine (AdN2, 1) was systematically investigated. The combined HPLC and MALDI-TOF analyses show that carbene addition by photochemical reaction afforded three isomers of Th@C 3v(8)-C82Ad and four isomers of U@C 2v(9)-C82Ad (Ad = adamantylidene), presenting notably higher reactivity than their lanthanide analogs. Among these novel EMF derivatives, Th@C 3v(8)-C82Ad(I, II, III) and U@C 2v(9)-C82Ad(I, II, III) were successfully isolated and were characterized by UV-vis-NIR spectroscopy. In particular, the molecular structures of first actinide fullerene derivatives, Th@C 3v(8)-C82Ad(I) and U@C 2v(9)-C82Ad(I), were unambiguously determined by single crystal X-ray crystallography, both of which show a [6,6]-open cage structure. In addition, isomerization of Th@C 3v(8)-C82Ad(II), Th@C 3v(8)-C82Ad(III), U@C 2v(9)-C82Ad(II) and U@C 2v(9)-C82Ad(III) was observed at room temperature. Computational studies suggest that the attached carbon atoms on the cages of both Th@C 3v(8)-C82Ad(I) and U@C 2v(9)-C82Ad(I) have the largest negative charges, thus facilitating the electrophilic attack. Furthermore, it reveals that, compared to their lanthanide analogs, Th@C 3v(8)-C82 and U@C 2v(9)-C82 have much closer metal-cage distance, increased metal-to-cage charge transfer, and strong metal-cage interactions stemming from the significant contribution of extended Th-5f and U-5f orbitals to the occupied molecular orbitals, all of which give rise to their unusual high reactivity. This study provides first insights into the exceptional chemical properties of actinide endohedral fullerenes, which pave ways for the future functionalization and application of these novel EMF compounds.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
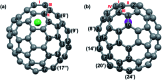

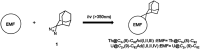
References
-
- Wang K. Liu X. Huang R. Wu C. Yang D. Hu X. Jiang X. Duchamp J. C. Dorn H. Priya S. ACS Energy Lett. 2019;4:1852–1861. doi: 10.1021/acsenergylett.9b01042. - DOI
-
- Zheng J.-P. Zhen M.-M. Wang C.-R. Shu C.-Y. Chin. J. Anal. Chem. 2012;40:1607–1615. doi: 10.3724/SP.J.1096.2012.20380. - DOI
-
- Jin P. Li Y. Magagula S. Chen Z. Coord. Chem. Rev. 2019;388:406–439. doi: 10.1016/j.ccr.2019.02.028. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

