Stabilization of hydrated AcIII cation: the role of superatom states in actinium-water bonding
- PMID: 34164034
- PMCID: PMC8179294
- DOI: 10.1039/d0sc02342f
Stabilization of hydrated AcIII cation: the role of superatom states in actinium-water bonding
Abstract
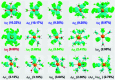
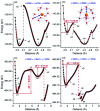
225Ac-based radiopharmaceuticals have the potential to become invaluable in designated cancer therapy. However, the limited understanding of the solution chemistry and bonding properties of actinium has hindered the development of existing and emerging targeted radiotherapeutics, which also poses a significant challenge in the discovery of new agents. Herein, we report the geometric and electronic structural properties of hydrated AcIII cations in the [AcIII(H2O) n ]3+ (n = 4-11) complexes in aqueous solution and gas-phase using density functional theory. We found that nine water molecules coordinated to the AcIII cation is the most stable complex due to an enhanced hydration Gibbs free energy. This complex adopts a closed-shell 18-electron configuration (1S 21P 61D 10) of a superatom state, which indicates a non-negligible covalent character and involves H2O → AcIII σ donation interaction between s-/p-/d-type atomic orbitals of the Ac atom and 2p atomic orbitals of the O atoms. Furthermore, potentially existing 10-coordinated complexes need to overcome an energy barrier (>0.10 eV) caused by hydrogen bonding to convert to 9-coordination. These results imply the importance of superatom states in actinide chemistry generally, and specifically in AcIII solution chemistry, and highlight the conversion mechanism between different coordination numbers.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Synthesis and Characterization of the Actinium Aquo Ion.ACS Cent Sci. 2017 Mar 22;3(3):176-185. doi: 10.1021/acscentsci.6b00356. Epub 2017 Feb 1. ACS Cent Sci. 2017. PMID: 28386595 Free PMC article.
-
Actinium coordination chemistry: A density functional theory study with monodentate and bidentate ligands.J Comput Chem. 2023 Jan 30;44(3):334-345. doi: 10.1002/jcc.26929. Epub 2022 Jun 6. J Comput Chem. 2023. PMID: 35668552
-
Computer-Assisted Design of Macrocyclic Chelators for Actinium-225 Radiotherapeutics.Inorg Chem. 2021 Jan 18;60(2):623-632. doi: 10.1021/acs.inorgchem.0c02432. Epub 2020 Nov 19. Inorg Chem. 2021. PMID: 33213142
-
Density functional theory studies of actinide(III) motexafins (An-Motex2+, An = Ac, Cm, Lr). Structure, stability, and comparison with lanthanide(III) motexafins.Inorg Chem. 2006 Apr 17;45(8):3444-51. doi: 10.1021/ic052128t. Inorg Chem. 2006. PMID: 16602805
-
A review of recent advancements in Actinium-225 labeled compounds and biomolecules for therapeutic purposes.Chem Biol Drug Des. 2023 Nov;102(5):1276-1292. doi: 10.1111/cbdd.14311. Epub 2023 Sep 15. Chem Biol Drug Des. 2023. PMID: 37715360 Review.
Cited by
-
Complexation of 3p-C-NETA with radiometal ions: A density functional theory study for targeted radioimmunotherapy.Heliyon. 2024 Jul 20;10(15):e34875. doi: 10.1016/j.heliyon.2024.e34875. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39144950 Free PMC article.
-
Estimating the Gibbs Hydration Energies of Actinium and Trans-Plutonium Actinides.Chemphyschem. 2023 Jan 17;24(2):e202200516. doi: 10.1002/cphc.202200516. Epub 2022 Nov 4. Chemphyschem. 2023. PMID: 36149643 Free PMC article.
-
Actinium chelation and crystallization in a macromolecular scaffold.Nat Commun. 2024 Jul 15;15(1):5741. doi: 10.1038/s41467-024-50017-5. Nat Commun. 2024. PMID: 39009580 Free PMC article.
-
225Ac-rHDL Nanoparticles: A Potential Agent for Targeted Alpha-Particle Therapy of Tumors Overexpressing SR-BI Proteins.Molecules. 2022 Mar 27;27(7):2156. doi: 10.3390/molecules27072156. Molecules. 2022. PMID: 35408554 Free PMC article.
References
LinkOut - more resources
Full Text Sources

