N-Heterocyclic carbene-carbodiimide (NHC-CDI) betaine adducts: synthesis, characterization, properties, and applications
- PMID: 34164037
- PMCID: PMC8179359
- DOI: 10.1039/d0sc06465c
N-Heterocyclic carbene-carbodiimide (NHC-CDI) betaine adducts: synthesis, characterization, properties, and applications
Abstract
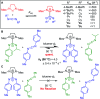
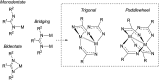
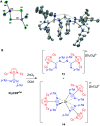
N-Heterocyclic carbenes (NHCs) are an important class of reactive organic molecules used as ligands, organocatalysts, and σ-donors in a variety of electroneutral ylide or betaine adducts with main-group compounds. An emerging class of betaine adducts made from the reaction of NHCs with carbodiimides (CDIs) form zwitterionic amidinate-like structures with tunable properties based on the highly modular NHC and CDI scaffolds. The adduct stability is controlled by the substituents on the CDI nitrogens, while the NHC substituents greatly affect the configuration of the adduct in the solid state. This Perspective is intended as a primer to these adducts, touching on their history, synthesis, characterization, and general properties. Despite the infancy of the field, NHC-CDI adducts have been applied as amidinate-type ligands for transition metals and nanoparticles, as junctions in zwitterionic polymers, and to stabilize distonic radical cations. These applications and potential future directions are discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no conflicts to declare.
Figures















Similar articles
-
An N-Heterocyclic-Carbene-Derived Distonic Radical Cation.Angew Chem Int Ed Engl. 2020 Mar 2;59(10):3952-3955. doi: 10.1002/anie.201915534. Epub 2020 Jan 30. Angew Chem Int Ed Engl. 2020. PMID: 31825136
-
Switchable Organocatalysis from N-heterocyclic Carbene-Carbodiimide Adducts with Tunable Release Temperature.Angew Chem Int Ed Engl. 2023 Nov 27;62(48):e202314376. doi: 10.1002/anie.202314376. Epub 2023 Oct 25. Angew Chem Int Ed Engl. 2023. PMID: 37824288
-
N-Heterocyclic Carbene-Carbodiimide ("NHC-CDI") Adduct or Zwitterionic-Type Neutral Amidinate-Supported Magnesium(II) and Zinc(II) Complexes.Inorg Chem. 2017 Aug 21;56(16):9535-9546. doi: 10.1021/acs.inorgchem.7b00879. Epub 2017 Aug 7. Inorg Chem. 2017. PMID: 28782943
-
BIAN-NHC Ligands in Transition-Metal-Catalysis: A Perfect Union of Sterically Encumbered, Electronically Tunable N-Heterocyclic Carbenes?Chemistry. 2021 Mar 8;27(14):4478-4499. doi: 10.1002/chem.202003923. Epub 2021 Jan 18. Chemistry. 2021. PMID: 32989914 Free PMC article. Review.
-
Application of N-heterocyclic carbene-Cu(I) complexes as catalysts in organic synthesis: a review.Beilstein J Org Chem. 2023 Sep 20;19:1408-1442. doi: 10.3762/bjoc.19.102. eCollection 2023. Beilstein J Org Chem. 2023. PMID: 37767335 Free PMC article. Review.
Cited by
-
NHC-CDI Betaine Adducts and Their Cationic Derivatives as Catalyst Precursors for Dichloromethane Valorization.J Org Chem. 2021 Dec 3;86(23):16725-16735. doi: 10.1021/acs.joc.1c01971. Epub 2021 Nov 1. J Org Chem. 2021. PMID: 34724613 Free PMC article.
-
Synthesis and reactivity of a six-membered heterocyclic 1,3-diphosphaallene.Chem Sci. 2024 Nov 29;16(3):1189-1196. doi: 10.1039/d4sc06371f. eCollection 2025 Jan 15. Chem Sci. 2024. PMID: 39664807 Free PMC article.
-
Exploring Mesoionic Imine-Carbodiimide (MII-CDI) Adducts: 1,3 H-Shift, N(I) Compounds and Guanidinate-Type Ligands.Angew Chem Int Ed Engl. 2025 Aug 18;64(34):e202502097. doi: 10.1002/anie.202502097. Epub 2025 Jul 16. Angew Chem Int Ed Engl. 2025. PMID: 40516060 Free PMC article.
-
The Genuine Carbene Conundrum.Chemistry. 2025 Aug 7;31(44):e202501600. doi: 10.1002/chem.202501600. Epub 2025 Jun 25. Chemistry. 2025. PMID: 40460197 Free PMC article. Review.
References
-
- Arduengo A. J. Harlow R. L. Kline M. A Stable Crystalline Carbene. J. Am. Chem. Soc. 1991;113:361–363. doi: 10.1021/ja00001a054. - DOI
-
- Igau A. Grutzmacher H. Baceiredo A. Bertrand G. Analogous α,α′-Bis-Carbenoid Triply Bonded Species: Synthesis of a Stable λ3-Phosphinocarbene-λ5-Phosphaacetylene. J. Am. Chem. Soc. 1988;110:6463–6466. doi: 10.1021/ja00227a028. - DOI
-
- Gardiner M. G. Ho C. C. Recent advances in bidentate bis(N-heterocyclic carbene) transition metal complexes and their applications in metal-mediated reactions. Coord. Chem. Rev. 2018;375:373–388. doi: 10.1016/j.ccr.2018.02.003. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

