Advances in anion binding and sensing using luminescent lanthanide complexes
- PMID: 34164038
- PMCID: PMC8179419
- DOI: 10.1039/d0sc05419d
Advances in anion binding and sensing using luminescent lanthanide complexes
Abstract
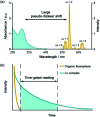
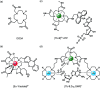
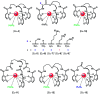
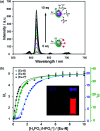
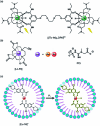
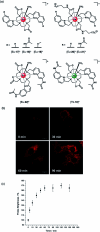
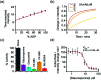
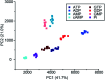
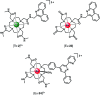
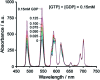
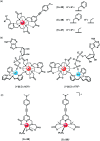
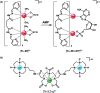
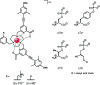
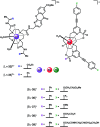
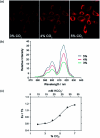
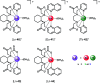
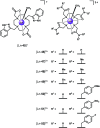
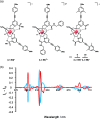
Luminescent lanthanide complexes have been actively studied as selective anion receptors for the past two decades. Ln(iii) complexes, particularly of europium(iii) and terbium(iii), offer unique photophysical properties that are very valuable for anion sensing in biological media, including long luminescence lifetimes (milliseconds) that enable time-gating methods to eliminate background autofluorescence from biomolecules, and line-like emission spectra that allow ratiometric measurements. By careful design of the organic ligand, stable Ln(iii) complexes can be devised for rapid and reversible anion binding, providing a luminescence response that is fast and sensitive, offering the high spatial resolution required for biological imaging applications. This review focuses on recent progress in the development of Ln(iii) receptors that exhibit sufficiently high anion selectivity to be utilised in biological or environmental sensing applications. We evaluate the mechanisms of anion binding and sensing, and the strategies employed to tune anion affinity and selectivity, through variations in the structure and geometry of the ligand. We highlight examples of luminescent Ln(iii) receptors that have been utilised to detect and quantify specific anions in biological media (e.g. human serum), monitor enzyme reactions in real-time, and visualise target anions with high sensitivity in living cells.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Enhancement of anion binding in lanthanide optical sensors.Acc Chem Res. 2013 Nov 19;46(11):2576-84. doi: 10.1021/ar400050t. Epub 2013 Sep 16. Acc Chem Res. 2013. PMID: 24032446 Free PMC article.
-
Application of lanthanide luminescence in probing enzyme activity.Chem Commun (Camb). 2018 Jun 19;54(50):6635-6647. doi: 10.1039/c8cc02824a. Chem Commun (Camb). 2018. PMID: 29790500
-
Luminescent lanthanide(III) complexes of DTPA-bis(amido-phenyl-terpyridine) for bioimaging and phototherapeutic applications.Spectrochim Acta A Mol Biomol Spectrosc. 2021 Jul 15;256:119709. doi: 10.1016/j.saa.2021.119709. Epub 2021 Mar 18. Spectrochim Acta A Mol Biomol Spectrosc. 2021. PMID: 33823402
-
Responsive Metal Complex Probes for Time-Gated Luminescence Biosensing and Imaging.Acc Chem Res. 2020 Jul 21;53(7):1316-1329. doi: 10.1021/acs.accounts.0c00172. Epub 2020 Jun 23. Acc Chem Res. 2020. PMID: 32574043 Review.
-
Luminescent Lanthanide Infinite Coordination Polymers for Ratiometric Sensing Applications.Molecules. 2025 Jan 18;30(2):396. doi: 10.3390/molecules30020396. Molecules. 2025. PMID: 39860266 Free PMC article. Review.
Cited by
-
Achieving Selectivity for Phosphate over Pyrophosphate in Ethanol with Iron(III)-Based Fluorescent Probes.JACS Au. 2022 Jun 25;2(7):1604-1609. doi: 10.1021/jacsau.2c00200. eCollection 2022 Jul 25. JACS Au. 2022. PMID: 35911450 Free PMC article.
-
Application of Machine Learning in Ethical Design of Autonomous Driving Crash Algorithms.Comput Intell Neurosci. 2022 Sep 24;2022:2938011. doi: 10.1155/2022/2938011. eCollection 2022. Comput Intell Neurosci. 2022. PMID: 36248938 Free PMC article.
-
Advance Progress on Luminescent Sensing of Nitroaromatics by Crystalline Lanthanide-Organic Complexes.Molecules. 2023 Jun 1;28(11):4481. doi: 10.3390/molecules28114481. Molecules. 2023. PMID: 37298958 Free PMC article. Review.
-
Expedient synthesis and luminescence sensing of the inositol pyrophosphate cellular messenger 5-PP-InsP5.Chem Sci. 2023 Apr 13;14(19):4979-4985. doi: 10.1039/d2sc06812e. eCollection 2023 May 17. Chem Sci. 2023. PMID: 37206391 Free PMC article.
-
A switch-on luminescent europium(iii) probe for selective and time-resolved detection of adenosine diphosphate (ADP).Chem Sci. 2025 Feb 19;16(13):5602-5612. doi: 10.1039/d4sc07188c. eCollection 2025 Mar 26. Chem Sci. 2025. PMID: 40028620 Free PMC article.
References
-
- Bünzli J.-C. G. J. Lumin. 2016;170:866–878. doi: 10.1016/j.jlumin.2015.07.033. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

