Diversified strategy for the synthesis of DNA-encoded oxindole libraries
- PMID: 34164048
- PMCID: PMC8179416
- DOI: 10.1039/d0sc06696f
Diversified strategy for the synthesis of DNA-encoded oxindole libraries
Abstract
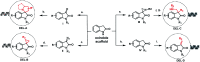
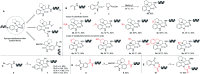
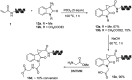
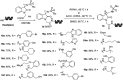
DNA-encoded library technology (DELT) employs DNA as a barcode to track the sequence of chemical reactions and enables the design and synthesis of libraries with billions of small molecules through combinatorial expansion. This powerful technology platform has been successfully demonstrated for hit identification and target validation for many types of diseases. As a highly integrated technology platform, DEL is capable of accelerating the translation of synthetic chemistry by using on-DNA compatible reactions or off-DNA scaffold synthesis. Herein, we report the development of a series of novel on-DNA transformations based on oxindole scaffolds for the design and synthesis of diversity-oriented DNA-encoded libraries for screening. Specifically, we have developed 1,3-dipolar cyclizations, cyclopropanations, ring-opening of reactions of aziridines and Claisen-Schmidt condensations to construct diverse oxindole derivatives. The majority of these transformations enable a diversity-oriented synthesis of DNA-encoded oxindole libraries which have been used in the successful hit identification for three protein targets. We have demonstrated that a diversified strategy for DEL synthesis could accelerate the application of synthetic chemistry for drug discovery.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Diversity-oriented synthesis as a tool to expand the chemical space of DNA-encoded libraries.Bioorg Med Chem. 2021 Jul 1;41:116218. doi: 10.1016/j.bmc.2021.116218. Epub 2021 May 15. Bioorg Med Chem. 2021. PMID: 34030087 Review.
-
Photochemical Methods Applied to DNA Encoded Library (DEL) Synthesis.Acc Chem Res. 2023 Feb 7;56(3):385-401. doi: 10.1021/acs.accounts.2c00778. Epub 2023 Jan 19. Acc Chem Res. 2023. PMID: 36656960 Free PMC article.
-
DNA-Encoded Libraries: Hydrazide as a Pluripotent Precursor for On-DNA Synthesis of Various Azole Derivatives.Chemistry. 2021 Jun 1;27(31):8214-8220. doi: 10.1002/chem.202100850. Epub 2021 Apr 27. Chemistry. 2021. PMID: 33811386
-
DNA-encoded chemical libraries: advancing beyond conventional small-molecule libraries.Acc Chem Res. 2014 Apr 15;47(4):1247-55. doi: 10.1021/ar400284t. Epub 2014 Mar 28. Acc Chem Res. 2014. PMID: 24673190
-
The expanding reaction toolkit for DNA-encoded libraries.Bioorg Med Chem Lett. 2021 Nov 1;51:128339. doi: 10.1016/j.bmcl.2021.128339. Epub 2021 Sep 1. Bioorg Med Chem Lett. 2021. PMID: 34478840 Review.
Cited by
-
A Small Molecule Selected from a DNA-Encoded Library of Natural Products That Binds to TNF-α and Attenuates Inflammation In Vivo.Adv Sci (Weinh). 2022 Jul;9(21):e2201258. doi: 10.1002/advs.202201258. Epub 2022 May 21. Adv Sci (Weinh). 2022. PMID: 35596609 Free PMC article.
-
Impact of organic chemistry conditions on DNA durability in the context of DNA-encoded library technology.iScience. 2023 Aug 9;26(9):107573. doi: 10.1016/j.isci.2023.107573. eCollection 2023 Sep 15. iScience. 2023. PMID: 37664608 Free PMC article.
-
Highly efficient on-DNA amide couplings promoted by micelle forming surfactants for the synthesis of DNA encoded libraries.Chem Sci. 2021 Jun 22;12(27):9475-9484. doi: 10.1039/d1sc03007h. eCollection 2021 Jul 14. Chem Sci. 2021. PMID: 34349922 Free PMC article.
-
Opportunities for Expanding Encoded Chemical Diversification and Improving Hit Enrichment in mRNA-Displayed Peptide Libraries.Chembiochem. 2022 Jun 20;23(12):e202100685. doi: 10.1002/cbic.202100685. Epub 2022 Feb 18. Chembiochem. 2022. PMID: 35100479 Free PMC article. Review.
References
-
- Schenone M. Dancik V. Wagner B. K. Clemons P. A. Nat. Chem. Biol. 2013;9:232–240. doi: 10.1038/nchembio.1199. - DOI - PMC - PubMed
- Finan C. Gaulton A. Kruger F. A. Lumbers R. T. Shah T. Engmann J. Galver L. Kelley R. Karlsson A. Santos R. Overington J. P. Hingorani A. D. Casas J. P. Sci. Transl. Med. 2017;9:eaag1166. doi: 10.1126/scitranslmed.aag1166. - DOI - PMC - PubMed
- Luo M. Jiao J. Wang R. BMC Bioinf. 2019;20:198. doi: 10.1186/s12859-019-2730-8. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

