FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients
- PMID: 34164344
- PMCID: PMC8216110
- DOI: 10.3389/fonc.2021.683419
FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients
Abstract
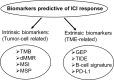
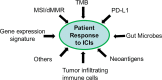
A patient's response to immune checkpoint inhibitors (ICIs) is a complex quantitative trait, and determined by multiple intrinsic and extrinsic factors. Three currently FDA-approved predictive biomarkers (progra1mmed cell death ligand-1 (PD-L1); microsatellite instability (MSI); tumor mutational burden (TMB)) are routinely used for patient selection for ICI response in clinical practice. Although clinical utility of these biomarkers has been demonstrated in ample clinical trials, many variables involved in using these biomarkers have poised serious challenges in daily practice. Furthermore, the predicted responders by these three biomarkers only have a small percentage of overlap, suggesting that each biomarker captures different contributing factors to ICI response. Optimized use of currently FDA-approved biomarkers and development of a new generation of predictive biomarkers are urgently needed. In this review, we will first discuss three widely used FDA-approved predictive biomarkers and their optimal use. Secondly, we will review four novel gene signature biomarkers: T-cell inflamed gene expression profile (GEP), T-cell dysfunction and exclusion gene signature (TIDE), melanocytic plasticity signature (MPS) and B-cell focused gene signature. The GEP and TIDE have shown better predictive performance than PD-L1, and PD-L1 or TMB, respectively. The MPS is superior to PD-L1, TMB, and TIDE. The B-cell focused gene signature represents a previously unexplored predictive biomarker to ICI response. Thirdly, we will highlight two combined predictive biomarkers: TMB+GEP and MPS+TIDE. These integrated biomarkers showed improved predictive outcomes compared to a single predictor. Finally, we will present a potential nucleic acid biomarker signature, allowing DNA and RNA biomarkers to be analyzed in one assay. This comprehensive signature could represent a future direction of developing robust predictive biomarkers, particularly for the cold tumors, for ICI response.
Keywords: FDA-approved biomarkers; PD-1; TMB; immune checkpoint inhibitors; predictive biomarkers.
Copyright © 2021 Wang, Tong, Zhang, Zhang, Buzdin, Mu, Yan, Zhao, Chang, Duhon, Zhou, Zhao, Chen and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Gascon M, Isla D, Cruellas M, Galvez EM, Lastra R, Ocariz M, et al. . Intratumoral Versus Circulating Lymphoid Cells as Predictive Biomarkers in Lung Cancer Patients Treated With Immune Checkpoint Inhibitors: Is the Easiest Path the Best One? Cells (2020) 9(6):1525–41. 10.3390/cells9061525 - DOI - PMC - PubMed
-
- Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. . Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov (2017) 7(2):188–201. 10.1158/2159-8290.CD-16-1223 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

