Clinical Use of the Self-Assembling Peptide RADA16: A Review of Current and Future Trends in Biomedicine
- PMID: 34164387
- PMCID: PMC8216384
- DOI: 10.3389/fbioe.2021.679525
Clinical Use of the Self-Assembling Peptide RADA16: A Review of Current and Future Trends in Biomedicine
Abstract
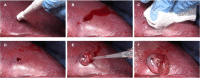
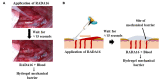
RADA16 is a synthetic peptide that exists as a viscous solution in an acidic formulation. In an acidic aqueous environment, the peptides spontaneously self-assemble into β-sheet nanofibers. Upon exposure and buffering of RADA16 solution to the physiological pH of biological fluids such as blood, interstitial fluid and lymph, the nanofibers begin physically crosslinking within seconds into a stable interwoven transparent hydrogel 3-D matrix. The RADA16 nanofiber hydrogel structure closely resembles the 3-dimensional architecture of native extracellular matrices. These properties make RADA16 formulations ideal topical hemostatic agents for controlling bleeding during surgery and to prevent post-operative rebleeding. A commercial RADA16 formulation is currently used for hemostasis in cardiovascular, gastrointestinal, and otorhinolaryngological surgical procedures, and studies are underway to investigate its use in wound healing and adhesion reduction. Straightforward application of viscous RADA16 into areas that are not easily accessible circumvents technical challenges in difficult-to-reach bleeding sites. The transparent hydrogel allows clear visualization of the surgical field and facilitates suture line assessment and revision. The shear-thinning and thixotropic properties of RADA16 allow its easy application through a narrow nozzle such as an endoscopic catheter. RADA16 hydrogels can fill tissue voids and do not swell so can be safely used in close proximity to pressure-sensitive tissues and in enclosed non-expandable regions. By definition, the synthetic peptide avoids potential microbiological contamination and immune responses that may occur with animal-, plant-, or mineral-derived topical hemostats. In vitro experiments, animal studies, and recent clinical experiences suggest that RADA16 nanofibrous hydrogels can act as surrogate extracellular matrices that support cellular behavior and interactions essential for wound healing and for tissue regenerative applications. In the future, the unique nature of RADA16 may also allow us to use it as a depot for precisely regulated drug and biopharmaceutical delivery.
Keywords: RADA16; endoscopy; hemostasis; nanofiber; self-assembling peptide hydrogel; surgery; tissue regeneration; wound healing.
Copyright © 2021 Sankar, O’Neill, Bagot D’Arc, Rebeca, Buffier, Aleksi, Fan, Matsuda, Gil and Spirio.
Conflict of interest statement
SS, KO’N, FR, MB, EA, MF, NM, EG, and LS are employees of 3-D Matrix, Ltd. MBD’A is a consultant for 3-D Matrix, Ltd.
Figures



References
-
- Araki T., Mitsuyama K., Yamasaki H., Morita M., Tsuruta K., Mori A., et al. (2021). Therapeutic potential of a self-assembling peptide hydrogel to treat colonic injuries associated with inflammatory bowel disease. J. Crohns. Colitis jjab033. 10.1093/ecco-jcc/jjab033 [Epub ahead of print]. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

