Convalescent Plasma Therapy for COVID-19: A Graphical Mosaic of the Worldwide Evidence
- PMID: 34164419
- PMCID: PMC8215127
- DOI: 10.3389/fmed.2021.684151
Convalescent Plasma Therapy for COVID-19: A Graphical Mosaic of the Worldwide Evidence
Abstract
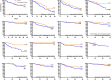
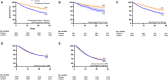
Convalescent plasma has been used worldwide to treat patients hospitalized with coronavirus disease 2019 (COVID-19) and prevent disease progression. Despite global usage, uncertainty remains regarding plasma efficacy, as randomized controlled trials (RCTs) have provided divergent evidence regarding the survival benefit of convalescent plasma. Here, we argue that during a global health emergency, the mosaic of evidence originating from multiple levels of the epistemic hierarchy should inform contemporary policy and healthcare decisions. Indeed, worldwide matched-control studies have generally found convalescent plasma to improve COVID-19 patient survival, and RCTs have demonstrated a survival benefit when transfused early in the disease course but limited or no benefit later in the disease course when patients required greater supportive therapies. RCTs have also revealed that convalescent plasma transfusion contributes to improved symptomatology and viral clearance. To further investigate the effect of convalescent plasma on patient mortality, we performed a meta-analytical approach to pool daily survival data from all controlled studies that reported Kaplan-Meier survival plots. Qualitative inspection of all available Kaplan-Meier survival data and an aggregate Kaplan-Meier survival plot revealed a directionally consistent pattern among studies arising from multiple levels of the epistemic hierarchy, whereby convalescent plasma transfusion was generally associated with greater patient survival. Given that convalescent plasma has a similar safety profile as standard plasma, convalescent plasma should be implemented within weeks of the onset of future infectious disease outbreaks.
Keywords: COVID-19; Kaplan–Meier analysis; SARS-CoV-2; convalescent plasma therapy; passive antibody transfer.
Copyright © 2021 Klassen, Senefeld, Senese, Johnson, Wiggins, Baker, van Helmond, Bruno, Pirofski, Shoham, Grossman, Henderson, Wright, Fairweather, Paneth, Carter, Casadevall and Joyner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- US Food and Drug Administration . Clinical Memorandum for the Emergency Use Authorization of COVID-19 Convalescent Plasma (2020). Available online at: https://www.fda.gov/media/141480/download (accessed April 29, 2021).
-
- United States Food and Drug Administration . Emergency Use Authorizations for Drug and Biological Products (2021). Available online at: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regula... (accessed April 29, 2021).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

