Blood Flow Within Bioengineered 3D Printed Vascular Constructs Using the Porcine Model
- PMID: 34164438
- PMCID: PMC8215112
- DOI: 10.3389/fcvm.2021.629313
Blood Flow Within Bioengineered 3D Printed Vascular Constructs Using the Porcine Model
Abstract
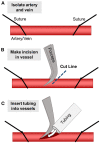
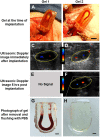
Recently developed biofabrication technologies are enabling the production of three-dimensional engineered tissues containing vascular networks which can deliver oxygen and nutrients across large tissue volumes. Tissues at this scale show promise for eventual regenerative medicine applications; however, the implantation and integration of these constructs in vivo remains poorly studied. Here, we introduce a surgical model for implantation and direct in-line vascular connection of 3D printed hydrogels in a porcine arteriovenous shunt configuration. Utilizing perfusable poly(ethylene glycol) diacrylate (PEGDA) hydrogels fabricated through projection stereolithography, we first optimized the implantation procedure in deceased piglets. Subsequently, we utilized the arteriovenous shunt model to evaluate blood flow through implanted PEGDA hydrogels in non-survivable studies. Connections between the host femoral artery and vein were robust and the patterned vascular channels withstood arterial pressure, permitting blood flow for 6 h. Our study demonstrates rapid prototyping of a biocompatible and perfusable hydrogel that can be implanted in vivo as a porcine arteriovenous shunt, suggesting a viable surgical approach for in-line implantation of bioprinted tissues, along with design considerations for future in vivo studies. We further envision that this surgical model may be broadly applicable for assessing whether biomaterials optimized for 3D printing and cell function can also withstand vascular cannulation and arterial blood pressure. This provides a crucial step toward generated transplantable engineered organs, demonstrating successful implantation of engineered tissues within host vasculature.
Keywords: 3D printed; bioengineered alternative tissue; porcine (pig) model; sterolithography; vascular constructs.
Copyright © 2021 Galván, Paulsen, Kinstlinger, Marini, Didelija, Yoeli, Grigoryan and Miller.
Conflict of interest statement
JSM and BG are cofounders of and hold an equity stake in the startup company Volumetric, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- McFadden TM, Duffy GP, Allen AB, Stevens HY, Schwarzmaier SM, Plesnila N, et al. . The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen-glycosaminoglycan scaffold in vivo. Acta Biomater. (2013) 9:9303–16. 10.1016/j.actbio.2013.08.014 - DOI - PubMed
LinkOut - more resources
Full Text Sources

