The association of adjusted plasma valproic acid concentration with CYP2C9 gene polymorphism in patients with epilepsy: a systematic review and meta-analysis
- PMID: 34164480
- PMCID: PMC8184431
- DOI: 10.21037/atm-21-1459
The association of adjusted plasma valproic acid concentration with CYP2C9 gene polymorphism in patients with epilepsy: a systematic review and meta-analysis
Abstract
Background: Valproic acid (VPA) is a common antiepileptic drug used to treat both generalized and partial epilepsy. Although there is increasing evidence to suggest that CYP2C9 gene polymorphisms are associated with interindividual variability of VPA metabolism, the results are debatable. Therefore, in the present study, we conducted a meta-analysis to evaluate the correlation between CYP2C9 gene polymorphisms and adjusted plasma VPA concentration.
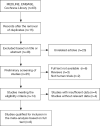
Methods: The EMBASE, MEDLINE, and Cochrane Library databases were searched to obtain relevant studies. Eligible articles were reviewed, and data extraction was performed. We calculated 95% confidence intervals (CIs) and mean differences (MDs) to assess the strength of the relationship of CYP2C9 gene polymorphisms with adjusted plasma VPA concentration.
Results: The meta-analysis included 6 studies involving 847 patients with epilepsy. The pooled analysis showed that the CYP2C9 A1075C (AA vs. AC) polymorphism was related to the adjusted plasma concentration of VPA (P=0.02, I2= 82%). Additionally, the AC phenotype statistically significantly increased the adjusted plasma VPA concentration in children compared with the mixed age subgroup (P=0.04, I2= 48%). A similar association was observed between the AC phenotype for Asians (P<0.00001, I2=0%) but not for Caucasians (P=0.34, I2=87%).
Discussion: Age might be a crucial covariate influencing the dosage-adjusted VPA concentration in patients with epilepsy. A reduced VPA dosage may be recommendable for children, particularly Asian children, who are CYP2C9 A1075C AC carriers. Further studies could provide high-quality evidence to confirm the correlation between VPA pharmacokinetics and CYP2C9 A1075C polymorphisms.
Keywords: CYP2C9; Valproic acid (VPA); genetic polymorphism; meta-analysis; pharmacokinetics.
2021 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-21-1459). The authors have no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
