Molecular landscape of head and neck cancer and implications for therapy
- PMID: 34164549
- PMCID: PMC8184465
- DOI: 10.21037/atm-20-6264
Molecular landscape of head and neck cancer and implications for therapy
Abstract
Head and neck squamous cell carcinomas (HNSCC) arising from the oral cavity, pharynx, and larynx constitute the 6th most common human cancer. Human papillomavirus (HPV)-positive tumours are distinct from HPV-negative counterparts, with HPV status affording clear clinical utility, prognostic benefit and better treatment outcomes. In contrast to their HPV-positive counterparts, HPV-negative tumours are characterized by high mutational load and chromosomal aberrations, with varying copy number alteration (CNA) profiles. HNSCC are distinct tumours at the chromosomal, gene and expression levels, with additional insight gained from immune profiling. Based on mutational analyses, HNSCC are categorized as HPV-positive, HPV-negative CNA-silent, and HPV-negative CNA-high tumours. Furthermore, gene expression profiling segregates these tumours into atypical, classical, basal, and mesenchymal, with clear differences observed between tumours of the oral cavity, oropharynx, hypopharynx and larynx. Additional immune profiling further classifies tumours as either immune-active or immune-exhausted. The clinical utility and impact of these tumour molecular subtypes however remains to be determined. HNSCC harbor high levels of somatic mutations. They display loss at 3p and 18q and gain at 3q and 8q, with mutations in CDKN2A, TP53, CCND1, EGFR, PIK3CA, PTEN, NOTCH1, NSD1, FAT1, AJUBA and KMT2D. Important pathways include the p53 and RB pathways which are involved in cell cycle control and are frequently lost in HPV-negative tumours, the WNT-β-catenin pathway related to the mesenchymal subtype and smoking etiology, and the PI3K pathway which includes the most common genetic alteration in HPV-positive HNSCC. Understanding the mutational, genomic and transcriptomic landscape of HNSCC has leveraged better therapeutic approaches to manage this group of diseases, and it is hoped that additional insight into the molecular subtypes of HNSCC and its specific subsites will further drive improved strategies to stratify and treat patients with this debilitating disease.
Keywords: Head and neck cancer; molecular landscape; oral cancer; squamous cell carcinoma; therapy.
2021 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6264). The series “Head and Neck Cancers – Disease Biology, Diagnostics, Prevention and Management” was commissioned by the editorial office without any funding or sponsorship. The author has no conflicts of interest to declare.
Figures
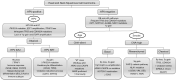
References
-
- Hashibe M, Brennan P, Benhamou S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. J Natl Cancer Inst 2007; 99:777-89. 10.1093/jnci/djk179 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
