Study design of a randomised, placebo-controlled trial of nintedanib in children and adolescents with fibrosing interstitial lung disease
- PMID: 34164554
- PMCID: PMC8215331
- DOI: 10.1183/23120541.00805-2020
Study design of a randomised, placebo-controlled trial of nintedanib in children and adolescents with fibrosing interstitial lung disease
Abstract
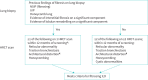
Childhood interstitial lung disease (chILD) comprises >200 rare respiratory disorders, with no currently approved therapies and variable prognosis. Nintedanib reduces the rate of forced vital capacity (FVC) decline in adults with progressive fibrosing interstitial lung diseases (ILDs). We present the design of a multicentre, prospective, double-blind, randomised, placebo-controlled clinical trial of nintedanib in patients with fibrosing chILD (1199-0337 or InPedILD; ClinicalTrials.gov: NCT04093024). Male or female children and adolescents aged 6-17 years (≥30; including ≥20 adolescents aged 12-17 years) with clinically significant fibrosing ILD will be randomised 2:1 to receive oral nintedanib or placebo on top of standard of care for 24 weeks (double-blind), followed by variable-duration nintedanib (open-label). Nintedanib dosing will be based on body weight-dependent allometric scaling, with single-step dose reductions permitted to manage adverse events. Eligible patients will have evidence of fibrosis on high-resolution computed tomography (within 12 months of their first screening visit), FVC ≥25% predicted, and clinically significant disease (Fan score of ≥3 or evidence of clinical progression over time). Patients with underlying chronic liver disease, significant pulmonary arterial hypertension, cardiovascular disease, or increased bleeding risk are ineligible. The primary endpoints are pharmacokinetics and the proportion of patients with treatment-emergent adverse events at week 24. Secondary endpoints include change in FVC% predicted from baseline, Pediatric Quality of Life Questionnaire, oxygen saturation, and 6-min walk distance at weeks 24 and 52. Additional efficacy and safety endpoints will be collected to explore long-term effects.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: R. Deterding reports scientific advisory and consulting fees paid to the University of Colorado, and manuscript preparation assistance from Boehringer Ingelheim Pharmaceuticals Inc., during the conduct of the study. Conflict of interest: M. Griese reports personal fees from Boehringer Ingelheim during the conduct of the study and grants from Boehringer Ingelheim outside the submitted work. Conflict of interest: G. Deutsch reports consulting fees paid to Seattle Children's Hospital by Boehringer Ingelheim during the conduct of the study. Conflict of interest: D. Warburton serves in an advisory role for Boehringer Ingelheim on the evaluation of nintedanib as a potential treatment for childhood ILD, and has received reimbursement for travel and consultation in this role. Conflict of interest: E.M. DeBoer reports consulting fees from Boehringer Ingelheim and Parexel, and consulting fees from and stock in EvoEndoscopy, outside the submitted work. Conflict of interest: S. Cunningham reports consultancy fees paid to the University of Edinburgh by Boehringer Ingelheim during the conduct of the study. Conflict of interest: A. Clement has nothing to disclose. Conflict of interest: N. Schwerk reports consulting fees from Boehringer Ingelheim outside the submitted work. Conflict of interest: K.R. Flaherty reports grants and personal fees from Boehringer Ingelheim, and personal fees from Roche/Genentech, Bellerophan, Respivant and Blade Therapeutics, outside the submitted work. Conflict of interest: K.K. Brown reports, outside the submitted work, grants from NHLBI, personal fees from Biogen and advisory board participation for Blade, Boehringer Ingelheim, Galapagos, Galecto, Genoa, Lifemax, MedImmune, OSIC (Open Source Imaging Consortium), Pliant, ProMetic, Third Pole, Theravance, Three Lakes Partners and Veracyte. Conflict of interest: F. Voss is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG. Conflict of interest: U. Schmid is an employee of Boehringer Ingelheim. Conflict of interest: R. Schlenker-Herceg is an employee of Boehringer Ingelheim. Conflict of interest: D. Verri is an employee of Boehringer Ingelheim Italia S.p.A. Conflict of interest: M. Dumistracel is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG. Conflict of interest: M. Schiwek is an employee of Boehringer Ingelheim Pharma GmbH & Co. KG. Conflict of interest: S. Stowasser is an employee of Boehringer Ingelheim International GmbH. Conflict of interest: K. Tetzlaff is an employee of Boehringer Ingelheim International GmbH. Conflict of interest: E. Clerisme-Beaty is an employee of Boehringer Ingelheim. Conflict of interest: L.R. Young reports personal fees for advisory board participation from Boehringer Ingelheim, and grants from the NIH, during the conduct of the study. All authors disclose third-party writing assistance contracted and funded by Boehringer Ingelheim International GmbH.
Figures


Similar articles
-
Nintedanib in children and adolescents with fibrosing interstitial lung diseases.Eur Respir J. 2023 Feb 2;61(2):2201512. doi: 10.1183/13993003.01512-2022. Print 2023 Feb. Eur Respir J. 2023. PMID: 36041751 Free PMC article. Clinical Trial.
-
Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial.Lancet Respir Med. 2020 May;8(5):453-460. doi: 10.1016/S2213-2600(20)30036-9. Epub 2020 Mar 5. Lancet Respir Med. 2020. PMID: 32145830 Clinical Trial.
-
Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial.Lancet Respir Med. 2020 Feb;8(2):147-157. doi: 10.1016/S2213-2600(19)30341-8. Epub 2019 Sep 29. Lancet Respir Med. 2020. PMID: 31578169 Clinical Trial.
-
Progressive fibrosing interstitial lung diseases: A new concept and indication of nintedanib.Mod Rheumatol. 2021 Jan;31(1):13-19. doi: 10.1080/14397595.2020.1826665. Mod Rheumatol. 2021. PMID: 32964766 Review.
-
Clinical use of nintedanib in patients with idiopathic pulmonary fibrosis.BMJ Open Respir Res. 2017 Jun 28;4(1):e000192. doi: 10.1136/bmjresp-2017-000192. eCollection 2017. BMJ Open Respir Res. 2017. PMID: 28883926 Free PMC article. Review.
Cited by
-
Diagnostic workup of childhood interstitial lung disease.Eur Respir Rev. 2023 Feb 21;32(167):220188. doi: 10.1183/16000617.0188-2022. Print 2023 Mar 31. Eur Respir Rev. 2023. PMID: 36813289 Free PMC article. Review.
-
Bibliometric analysis of the pirfenidone and nintedanib in interstitial lung diseases.Heliyon. 2024 Apr 15;10(8):e29266. doi: 10.1016/j.heliyon.2024.e29266. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38655311 Free PMC article.
-
Minimal important difference in childhood interstitial lung diseases.Thorax. 2023 May;78(5):476-483. doi: 10.1136/thorax-2022-219206. Epub 2022 Dec 26. Thorax. 2023. PMID: 36572533 Free PMC article.
-
Nintedanib in children and adolescents with fibrosing interstitial lung diseases.Eur Respir J. 2023 Feb 2;61(2):2201512. doi: 10.1183/13993003.01512-2022. Print 2023 Feb. Eur Respir J. 2023. PMID: 36041751 Free PMC article. Clinical Trial.
-
Lung Inflammation in STING-Associated Vasculopathy with Onset in Infancy (SAVI).Cells. 2022 Jan 18;11(3):318. doi: 10.3390/cells11030318. Cells. 2022. PMID: 35159128 Free PMC article. Review.
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
