Clinical, Laboratory, and Radiologic Characteristics of Patients With Initial False-Negative Severe Acute Respiratory Syndrome Coronavirus 2 Nucleic Acid Amplification Test Results
- PMID: 34164560
- PMCID: PMC7717411
- DOI: 10.1093/ofid/ofaa559
Clinical, Laboratory, and Radiologic Characteristics of Patients With Initial False-Negative Severe Acute Respiratory Syndrome Coronavirus 2 Nucleic Acid Amplification Test Results
Abstract
Background: Concerns about false-negative (FN) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleic acid amplification tests (NAATs) have prompted recommendations for repeat testing if suspicion for coronavirus disease 2019 (COVID-19) infection is moderate to high. However, the frequency of FNs and patient characteristics associated with FNs are poorly understood.
Methods: We retrospectively reviewed test results from 15 011 adults who underwent ≥1 SARS-CoV-2 NAATs; 2699 had an initial negative NAAT and repeat testing. We defined FNs as ≥1 negative NAATs followed by a positive NAAT within 14 days during the same episode of illness. We stratified subjects with FNs by duration of symptoms before the initial FN test (≤5 days versus >5 days) and examined their clinical, radiologic, and laboratory characteristics.
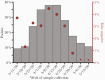
Results: Sixty of 2699 subjects (2.2%) had a FN result during the study period. The weekly frequency of FNs among subjects with repeat testing peaked at 4.4%, coinciding with peak NAAT positivity (38%). Most subjects with FNs had symptoms (52 of 60; 87%) and chest radiography (19 of 32; 59%) consistent with COVID-19. Of the FN NAATs, 18 of 60 (30%) were performed early (ie, ≤1 day of symptom onset), and 18 of 60 (30%) were performed late (ie, >7 days after symptom onset) in disease. Among 17 subjects with 2 consecutive FNs on NP NAATs, 9 (53%) provided lower respiratory tract (LRT) specimens for testing, all of which were positive.
Conclusions: Our findings support repeated NAATs among symptomatic patients, particularly during periods of higher COVID-19 incidence. The LRT testing should be prioritized to increase yield among patients with high clinical suspicion for COVID-19.
Keywords: COVID-19 testing; coronavirus; false-negative.
© The Author(s) 2020. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures


References
-
- Centers for Disease Control and Prevention. Coronavirus (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/index.html. Accessed 3 July 2020.
-
- Infectious Diseases Society of America. Guidelines on the diagnosis of COVID-19. Available at: https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnost.... Accessed 5 July 2020. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

