The role of HCFC1 in syndromic and non-syndromic intellectual disability
- PMID: 34164576
- PMCID: PMC8218923
- DOI: 10.18103/mra.v8i6.2122
The role of HCFC1 in syndromic and non-syndromic intellectual disability
Abstract
Mutations in the HCFC1 gene are associated with cases of syndromic (cblX) and non-syndromic intellectual disability. Syndromic individuals present with severe neurological defects including intractable epilepsy, facial dysmorphia, and intellectual disability. Non-syndromic individuals have also been described and implicate a role for HCFC1 during brain development. The penetrance of phenotypes and the presence of an overall syndrome is associated with the location of the mutation within the HCFC1 protein. Thus, one could hypothesize that the positioning of HCFC1 mutations lead to different neurological phenotypes that include but are not restricted to intellectual disability. The HCFC1 protein is comprised of multiple domains that function in cellular proliferation/metabolism. Several reports of HCFC1 disease variants have been identified, but a comprehensive review of each variant and its associated phenotypes has not yet been compiled. Here we perform a detailed review of HCFC1 function, model systems, variant location, and accompanying phenotypes to highlight current knowledge and the future status of the field.
Keywords: HCFC1; cblX; functional analysis; intellectual disability.
Conflict of interest statement
Conflicts of Interest Authors report no conflicts of interest
Figures
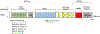
References
Grants and funding
LinkOut - more resources
Full Text Sources
