T cells mediate cell non-autonomous arterial ageing in mice
- PMID: 34164826
- PMCID: PMC8425389
- DOI: 10.1113/JP281698
T cells mediate cell non-autonomous arterial ageing in mice
Abstract
Key points: Increased large artery stiffness and impaired endothelium-dependent dilatation occur with advanced age. We sought to determine whether T cells mechanistically contribute to age-related arterial dysfunction. We found that old mice exhibited greater proinflammatory T cell accumulation around both the aorta and mesenteric arteries. Pharmacologic depletion or genetic deletion of T cells in old mice resulted in ameliorated large artery stiffness and greater endothelium-dependent dilatation compared with mice with T cells intact.
Abstract: Ageing of the arteries is characterized by increased large artery stiffness and impaired endothelium-dependent dilatation. T cells contribute to hypertension in acute rodent models but whether they contribute to chronic age-related arterial dysfunction is unknown. To determine whether T cells directly mediate age-related arterial dysfunction, we examined large elastic artery and resistance artery function in young (4-6 months) and old (22-24 months) wild-type mice treated with anti-CD3 F(ab'2) fragments to deplete T cells (150 μg, i.p. every 7 days for 28 days) or isotype control fragments. Old mice exhibited greater numbers of T cells in both aorta and mesenteric vasculature when compared with young mice. Old mice treated with anti-CD3 fragments exhibited depletion of T cells in blood, spleen, aorta and mesenteric vasculature. Old mice also exhibited greater numbers of aortic and mesenteric IFN-γ and TNF-α-producing T cells when compared with young mice. Old control mice exhibited greater large artery stiffness and impaired resistance artery endothelium-dependent dilatation in comparison with young mice. In old mice, large artery stiffness was ameliorated with anti-CD3 treatment. Anti-CD3-treated old mice also exhibited greater endothelium-dependent dilatation than age-matched controls. We also examined arterial function in young and old Rag-1-/- mice, which lack lymphocytes. Rag-1-/- mice exhibited blunted increases in large artery stiffness with age compared with wild-type mice. Old Rag-1-/- mice also exhibited greater endothelium-dependent dilatation compared with old wild-type mice. Collectively, these results demonstrate that T cells play an important role in age-related arterial dysfunction.
Keywords: aorta; endothelium; immune system; lymphocytes; mesentery; vascular.
© 2021 The Authors. The Journal of Physiology © 2021 The Physiological Society.
Figures

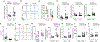

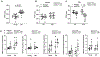
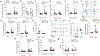

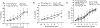

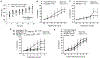
Comment in
-
Could angiotensin-II induced T-cell senescence exacerbate age-related vascular dysfunction?J Physiol. 2022 Apr;600(8):1821-1823. doi: 10.1113/JP282581. Epub 2022 Mar 20. J Physiol. 2022. PMID: 35238408 Free PMC article. No abstract available.
References
-
- Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR & Wilkinson IB (2014). Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 63, 636–646. - PMC - PubMed
-
- Blackwell KA, Sorenson JP, Richardson DM, Smith LA, Suda O, Nath K & Katusic ZS (2004). Mechanisms of aging-induced impairment of endothelium-dependent relaxation: role of tetrahydrobiopterin. Am J Physiol Heart Circ Physiol 287, H2448–2453. - PubMed
-
- Callahan JE, Kappler JW & Marrack P (1993). Unexpected expansions of CD8-bearing cells in old mice. J Immunol 151, 6657–6669. - PubMed
-
- Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, Kim HA, Krishnan SM, Lewis CV, Salimova E, Tipping P, Vinh A, Samuel CS, Peter K, Guzik TJ, Kyaw TS, Toh BH, Bobik A & Drummond GR (2015). Obligatory Role for B Cells in the Development of Angiotensin II-Dependent Hypertension. Hypertension 66, 1023–1033. - PubMed
Publication types
MeSH terms
Grants and funding
- I01 BX004492/BX/BLRD VA/United States
- K01 AG061271/AG/NIA NIH HHS/United States
- R01 AG060395/NH/NIH HHS/United States
- K01 AG061271/NH/NIH HHS/United States
- T32 HL139451/HL/NHLBI NIH HHS/United States
- R01 AG077751/AG/NIA NIH HHS/United States
- R01 AG050238/AG/NIA NIH HHS/United States
- S10 RR026802/RR/NCRR NIH HHS/United States
- R01 AG050238/NH/NIH HHS/United States
- R01 AG048366/AG/NIA NIH HHS/United States
- R01 AG060395/AG/NIA NIH HHS/United States
- R00 AT010017/AT/NCCIH NIH HHS/United States
- P30 CA042014/CA/NCI NIH HHS/United States
- R01 AG048366/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources

