Transient association between semen exposure and biomarkers of genital inflammation in South African women at risk of HIV infection
- PMID: 34164927
- PMCID: PMC8223121
- DOI: 10.1002/jia2.25766
Transient association between semen exposure and biomarkers of genital inflammation in South African women at risk of HIV infection
Abstract
Introduction: Semen induces mucosal changes in the female reproductive tract to improve pregnancy outcomes. Since semen-induced alterations are likely short-lived and genital inflammation is linked to HIV acquisition in women, we investigated the contribution of recent semen exposure on biomarkers of genital inflammation in women at high HIV risk and the persistence of these associations.
Methods: We assessed stored genital specimens from 152 HIV-negative KwaZulu-Natal women who participated in the CAPRISA 008 trial between November 2012 and October 2014. During the two-year study period, 651 vaginal specimens were collected biannually (mean five samples per woman). Cervicovaginal lavage (CVL) was screened for prostate-specific antigen (PSA) by ELISA, whereas Y-chromosome DNA (YcDNA) detection and quantification were conducted by RT-PCR, representing semen exposure within 48 hours (PSA+YcDNA+) and semen exposure within three to fifteen days (PSA-YcDNA+). Soluble protein concentrations were measured in CVLs by multiplexed ELISA. T-cell frequencies were assessed in cytobrushes by flow-cytometry, and vulvovaginal swabs were used to detect common vaginal microbes by PCR. Linear mixed models adjusting for factors associated with genital inflammation and HIV risk were used to assess the impact of semen exposure on biomarkers of inflammation over multiple visits.
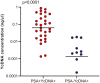
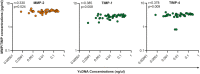
Results: Here, 19% (125/651) of CVLs were PSA+YcDNA+, 14% (93/651) were PSA-YcDNA+ and 67% (433/651) were PSA-YcDNA-. Semen exposure was associated with how often women saw their partners, the frequency of vaginal sex in the past month, HSV-2 antibody detection, current gonorrhoea infection and Nugent Score. Both PSA detection (PSA+YcDNA+) and higher cervicovaginal YcDNA concentrations predicted increases in several cytokines, barrier-related proteins (MMP-2, TIMP-1 and TIMP-4) and activated CD4+CCR5+HLA-DR+ T cells (β = 0.050; CI 0.001 to 0.098; p = 0.046) and CD4+HLA-DR+ T cells (β = 0.177; CI 0.016 to 0.339; p = 0.032) respectively. PSA detection was specifically associated with raised pro-inflammatory cytokines (including IL-6, TNF-α, IP-10 and RANTES), and with the detection of BVAB2 (OR = 1.755; CI 1.116 to 2.760; p = 0.015), P. bivia (OR = 1.886; CI 1.102 to 3.228; p = 0.021) and Gardnerella vaginalis (OR = 1.815; CI 1.093 to 3.015; p = 0.021).
Conclusions: More recent semen exposure was associated with raised levels of inflammatory biomarkers and the detection of BV-associated microbes, which declined by three to fifteen days of post-exposure. Although transient, semen-induced alterations may have implications for HIV susceptibility in women.
Keywords: HIV; Y-chromosome DNA; cytokines; female genital inflammation; prostate-specific antigen; semen.
© 2021 The Authors. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Figures





Similar articles
-
The Impact of Semen Exposure on the Immune and Microbial Environments of the Female Genital Tract.Front Reprod Health. 2020 Nov 9;2:566559. doi: 10.3389/frph.2020.566559. eCollection 2020. Front Reprod Health. 2020. PMID: 36304709 Free PMC article.
-
Recent Semen Exposure Impacts the Cytokine Response and Bacterial Vaginosis in Women.Front Immunol. 2021 Jun 9;12:695201. doi: 10.3389/fimmu.2021.695201. eCollection 2021. Front Immunol. 2021. PMID: 34177961 Free PMC article.
-
Defining characteristics of genital health in South African adolescent girls and young women at high risk for HIV infection.PLoS One. 2019 Apr 4;14(4):e0213975. doi: 10.1371/journal.pone.0213975. eCollection 2019. PLoS One. 2019. PMID: 30947260 Free PMC article.
-
Semen: A modulator of female genital tract inflammation and a vector for HIV-1 transmission.Am J Reprod Immunol. 2021 Nov;86(5):e13478. doi: 10.1111/aji.13478. Epub 2021 Jun 16. Am J Reprod Immunol. 2021. PMID: 34077596 Free PMC article. Review.
-
The use of PSA as a biomarker of recent semen exposure in female reproductive health studies.J Reprod Immunol. 2021 Nov;148:103381. doi: 10.1016/j.jri.2021.103381. Epub 2021 Sep 9. J Reprod Immunol. 2021. PMID: 34563757 Review.
Cited by
-
Considerations for Choosing Soluble Immune Markers to Determine Safety of Novel Vaginal Products.Front Reprod Health. 2022 May 17;4:899277. doi: 10.3389/frph.2022.899277. eCollection 2022. Front Reprod Health. 2022. PMID: 36303630 Free PMC article. Review.
-
Starting to have sexual intercourse is associated with increases in cervicovaginal immune mediators in young women: a prospective study and meta-analysis.Elife. 2022 Oct 25;11:e78565. doi: 10.7554/eLife.78565. Elife. 2022. PMID: 36281966 Free PMC article.
-
A Noninvasive Method to Sample Immune Cells in the Lower Female Genital Tract Using Menstrual Discs.Immunohorizons. 2024 Feb 1;8(2):182-192. doi: 10.4049/immunohorizons.2300105. Immunohorizons. 2024. PMID: 38386594 Free PMC article.
References
-
- Sharkey DJ, Tremellen KP, Jasper MJ, Gemzell‐Danielsson K, Robertson SA. Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus. J Immunol. 2012;188(5):2445–54. - PubMed
-
- Robertson SA, Guerin LR, Moldenhauer LM, Hayball JD. Activating T regulatory cells for tolerance in early pregnancy – the contribution of seminal fluid. J Reprod Immunol. 2009;83(1–2):109–16. - PubMed
-
- Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV‐susceptible target cells. Mucosal Immunol. 2016;9(1):194–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

