Mandibular advancement device use in obstructive sleep apnea: ORCADES study 5-year follow-up data
- PMID: 34165074
- PMCID: PMC8656912
- DOI: 10.5664/jcsm.9308
Mandibular advancement device use in obstructive sleep apnea: ORCADES study 5-year follow-up data
Abstract
Study objectives: Mandibular advancement devices (MADs) are an alternative to continuous positive airway pressure for the management of obstructive sleep apnea (OSA). The ORthèse d'avanCée mAndibulaire dans le traitement en DEuxième intention du SAHOS sévère (ORCADES) study is investigating the long-term effectiveness of MAD therapy in patients with OSA who refused or were intolerant of continuous positive airway pressure. Five-year follow-up data are presented.
Methods: Data were available in 172 of 331 patients treated with a custom-made computer-aided design/computer-aided manufacturing biblock MAD (Narval CC; ResMed, Saint-Priest, France). The primary end point was treatment success (≥50% decrease in apnea-hypopnea index from baseline).
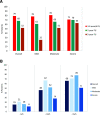
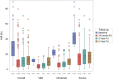
Results: Five-year treatment success rates were 52% overall and 25%, 52%, and 63%, respectively, in patients with mild, moderate, or severe OSA. This reflects a decline over time vs 3-6 months (79% overall) and 2 years (68%). Rates declined in all patient subgroups but to the greatest extent in patients with mild OSA. The slight worsening of respiratory parameters over time was not associated with any relevant changes in sleepiness and symptoms. Moderate or severe OSA at baseline, treatment success at 3-6 months, and no previous continuous positive airway pressure use were significant independent predictors of 5-year treatment success on multivariate analysis. No new safety signals emerged during long-term follow-up. The proportion of patients using their MAD for ≥4 h/night on ≥4 days/wk was 93.3%; 91.3% of patients reported device use of ≥6 h/night at 5 years. At 5-year follow-up, 96.5% of patients reported that they wanted to continue MAD therapy.
Conclusions: Long-term MAD therapy remained effective after 5 years in >50% of patients, with good levels of patient satisfaction and adherence.
Citation: Vecchierini MF, Attali V, Collet JM, et al. Mandibular advancement device use in obstructive sleep apnea: ORCADES study 5-year follow-up data. J Clin Sleep Med. 2021;17(8):1695-1705.
Keywords: adherence; apnea-hypopnea index; mandibular advancement device; obstructive sleep apnea.
© 2021 American Academy of Sleep Medicine.
Figures


Comment in
-
Is it the time to expect long-term outcome data in addition to follow-up data for sleep apnea interventions?J Clin Sleep Med. 2021 Aug 1;17(8):1519-1520. doi: 10.5664/jcsm.9464. J Clin Sleep Med. 2021. PMID: 34047692 Free PMC article.
References
-
- Chan AS , Sutherland K , Schwab RJ , et al. . The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea . Thorax . 2010. ; 65 ( 8 ): 726 – 732 . - PubMed
-
- Kato J , Isono S , Tanaka A , et al. . Dose-dependent effects of mandibular advancement on pharyngeal mechanics and nocturnal oxygenation in patients with sleep-disordered breathing . Chest . 2000. ; 117 ( 4 ): 1065 – 1072 . - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

