Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non-Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial
- PMID: 34165501
- PMCID: PMC8227450
- DOI: 10.1001/jamaoncol.2021.1910
Effect of Postoperative Radiotherapy for Patients With pIIIA-N2 Non-Small Cell Lung Cancer After Complete Resection and Adjuvant Chemotherapy: The Phase 3 PORT-C Randomized Clinical Trial
Abstract
Importance: The role of postoperative radiotherapy (PORT) has not been well defined in resected pIIIA-N2 non-small cell lung cancer (NSCLC).
Objective: To evaluate the effect of PORT using modern techniques on survival and safety in patients with pIIIA-N2 NSCLC after complete resection and adjuvant chemotherapy.
Design, setting, and participants: The PORT-C randomized clinical trial was conducted in 394 patients with pIIIA-N2 NSCLC treated with complete resection and 4 cycles of platinum-based chemotherapy between January 2009 and December 2017. Data were analyzed between March 2019 and December 2020.
Interventions: Patients were randomized equally into the PORT arm (n = 202) or the observation arm (n = 192). The total dose of PORT was 50 Gy.
Main outcomes and measures: The primary end point was disease-free survival (DFS). Secondary end points included overall survival (OS), locoregional recurrence-free survival (LRFS), distant metastasis-free survival, and toxic effects.
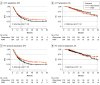
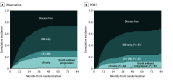
Results: In total, 394 patients were enrolled and 364 were eligible, with a median (range) age of 55 (25-70) years. There were 202 (55.5%) male and 162 (44.5%) female patients. The median follow-up was 46.0 (95% CI, 41.9-51.4) months, and 230 DFS events were reported. There were 184 patients in the PORT arm and 180 patients in the observation arm. The 3-year DFS rates were 40.5% with PORT vs 32.7% with observation (median, 22.1 vs 18.6 months), and the difference in DFS was not statistically significant without adjustment (hazard ratio [HR], 0.84; 95% CI, 0.65-1.09; P = .20), though it was significant with preplanned yet exploratory analysis (stratified analysis by the number of detected lymph nodes and positive lymph nodes, HR, 0.75; log-rank P = .04). The 3-year OS rates were 78.3% vs 82.8% (HR, 1.02; P = .93), and LRFS was 66.5% vs 59.7% (HR, 0.71; 95% CI, 0.51-0.97; P = .03), respectively. For 310 per-protocol patients (140 with PORT and 170 with observation), PORT significantly improved DFS (42.8% vs 30.6%; HR, 0.75; 95% CI, 0.57-1.00; P = .05) but not OS (HR, 0.83; 95% CI, 0.53-1.30; P = .41). The 3-year local recurrence only rates were 9.5% and 18.3% in the 2 arms, respectively (Fine-Gray HR, 0.55; Gray test P = .04). No radiotherapy-related grade 4 or 5 adverse event was observed.
Conclusions and relevance: In this phase 3 randomized clinical trial of patients with pIIIA-N2 NSCLC after complete resection and adjuvant chemotherapy, PORT did not improve DFS. Further studies exploring patients who might best benefit from PORT are needed.
Trial registration: ClinicalTrials.gov Identifier: NCT00880971.
Conflict of interest statement
Figures

References
-
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719-727. doi: 10.1016/S1470-2045(06)70804-X - DOI - PubMed

