Confirmed 6-Month Disability Improvement and Worsening Correlate with Long-term Disability Outcomes in Alemtuzumab-Treated Patients with Multiple Sclerosis: Post Hoc Analysis of the CARE-MS Studies
- PMID: 34165694
- PMCID: PMC8571457
- DOI: 10.1007/s40120-021-00262-3
Confirmed 6-Month Disability Improvement and Worsening Correlate with Long-term Disability Outcomes in Alemtuzumab-Treated Patients with Multiple Sclerosis: Post Hoc Analysis of the CARE-MS Studies
Abstract
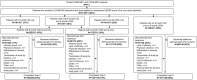
Introduction: In the 2-year CARE-MS trials (NCT00530348; NCT00548405) in patients with relapsing-remitting multiple sclerosis, alemtuzumab showed superior efficacy versus subcutaneous interferon beta-1a. Efficacy was maintained in two consecutive extensions (NCT00930553; NCT02255656). This post hoc analysis compared disability outcomes over 9 years among alemtuzumab-treated patients according to whether they experienced confirmed disability improvement (CDI) or worsening (CDW) or neither CDI nor CDW.
Methods: CARE-MS patients were randomized to receive two alemtuzumab courses (12 mg/day; 5 days at baseline; 3 days at 12 months), with additional as-needed 3-day courses in the extensions. CDI or CDW were defined as ≥ 1.0-point decrease or increase, respectively, in Expanded Disability Status Scale (EDSS) score from core study baseline confirmed over 6 months, assessed in patients with baseline EDSS score ≥ 2.0. Improved or stable EDSS scores were defined as ≥ 1-point decrease or ≤ 0.5-point change (either direction), respectively, from core study baseline. Functional systems (FS) scores were also assessed.
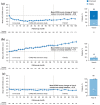
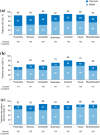
Results: Of 511 eligible patients, 43% experienced CDI and 34% experienced CDW at any time through year 9 (patients experiencing both CDI and CDW were counted in each individual group); 29% experienced neither CDI nor CDW. At year 9, patients with CDI had a -0.58-point mean EDSS score change from baseline; 88% had stable or improved EDSS scores. Improvements occurred across all FS, primarily in sensory, pyramidal, and cerebellar domains. Patients with CDW had a +1.71-point mean EDSS score change; 16% had stable or improved EDSS scores. Patients with neither CDI nor CDW had a -0.10-point mean EDSS score change; 98% had stable or improved EDSS scores.
Conclusion: CDI achievement at any point during the CARE-MS studies was associated with improved disability at year 9, highlighting the potential of alemtuzumab to change the multiple sclerosis course. Conversely, CDW at any point was associated with worsened disability at year 9.
Keywords: Alemtuzumab; Confirmed disability improvement; Confirmed disability worsening; Disease-modifying therapy; Expanded Disability Status Scale; Functional systems; Multiple sclerosis.
© 2021. The Author(s).
Figures