Continuous nasogastric milk feeding versus intermittent bolus milk feeding for preterm infants less than 1500 grams
- PMID: 34165778
- PMCID: PMC8223964
- DOI: 10.1002/14651858.CD001819.pub3
Continuous nasogastric milk feeding versus intermittent bolus milk feeding for preterm infants less than 1500 grams
Abstract
Background: Milk feedings can be given via nasogastric tube either intermittently, typically over 10 to 20 minutes every two or three hours, or continuously, using an infusion pump. Although the theoretical benefits and risks of each method have been proposed, their effects on clinically important outcomes remain uncertain. OBJECTIVES: To examine the evidence regarding the effectiveness of continuous versus intermittent bolus tube feeding of milk in preterm infants less than 1500 grams.
Search methods: We used the standard search strategy of Cochrane Neonatal to run comprehensive searches in the Cochrane Central Register of Controlled Trials (CENTRAL 2020, Issue 7) in the Cochrane Library; Ovid MEDLINE and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Daily and Versions; and CINAHL (Cumulative Index to Nursing and Allied Health Literature) on 17 July 2020. We also searched clinical trials databases and the reference lists of retrieved articles for randomised controlled trials (RCTs) and quasi-RCTs.
Selection criteria: We included RCTs and quasi-RCTs comparing continuous versus intermittent bolus nasogastric milk feeding in preterm infants less than 1500 grams.
Data collection and analysis: Two review authors independently assessed all trials for relevance and risk of bias. We used the standard methods of Cochrane Neonatal to extract data. We used the GRADE approach to assess the certainty of evidence. Primary outcomes were: age at full enteral feedings; feeding intolerance; days to regain birth weight; rate of gain in weight, length and head circumference; and risk of necrotising enterocolitis (NEC).
Main results: We included nine randomised trials (919 infants) in this updated Cochrane Review. One study is awaiting classification. Seven of the nine included trials reported data from infants with a maximum weight of between 1000 grams and 1400 grams. Two of the nine trials included infants weighing up to 1500 grams. Type(s) of milk feeds varied, including human milk (either mother's own milk or pasteurised donor human milk), preterm formula, or mixed feeding regimens. In some instances, preterm formula was initially diluted. Earlier studies also used water to initiate feedings. We judged six trials as unclear or high risk of bias for random sequence generation. We judged four trials as unclear for allocation concealment. We judged all trials as high risk of bias for blinding of care givers, and seven as unclear or high risk of bias for blinding of outcome assessors. We downgraded the certainty of evidence for imprecision, due to low numbers of participants in the trials, and/or wide 95% confidence intervals, and/or for risk of bias. Continuous compared to intermittent bolus (nasogastric and orogastric tube) milk feeding Babies receiving continuous feeding may reach full enteral feeding almost one day later than babies receiving intermittent feeding (mean difference (MD) 0.84 days, 95% confidence interval (CI) -0.13 to 1.81; 7 studies, 628 infants; low-certainty evidence). It is uncertain if there is any difference between continuous feeding and intermittent feeding in terms of number of days of feeding interruptions (MD -3.00 days, 95% CI -9.50 to 3.50; 1 study, 171 infants; very low-certainty evidence). It is uncertain if continuous feeding has any effect on days to regain birth weight (MD -0.38 days, 95% CI -1.16 to 0.41; 6 studies, 610 infants; low-certainty evidence). The certainty of evidence is low and the 95% confidence interval is consistent with possible benefit and possible harm. It is uncertain if continuous feeding has any effect on rate of gain in weight compared with intermittent feeding (standardised mean difference (SMD) 0.09, 95% CI -0.27 to 0.46; 5 studies, 433 infants; very low-certainty evidence). Continuous feeding may result in little to no difference in rate of gain in length compared with intermittent feeding (MD 0.02 cm/week, 95% CI -0.04 to 0.08; 5 studies, 433 infants; low-certainty evidence). Continuous feeding may result in little to no difference in rate of gain in head circumference compared with intermittent feeding (MD 0.01 cm/week, 95% CI -0.03 to 0.05; 5 studies, 433 infants; low-certainty evidence). It is uncertain if continuous feeding has any effect on the risk of NEC compared with intermittent feeding (RR 1.19, 95% CI 0.67 to 2.11; 4 studies, 372 infants; low-certainty evidence). The certainty of evidence is low and the 95% confidence interval is consistent with possible benefit and possible harm.
Authors' conclusions: Although babies receiving continuous feeding may reach full enteral feeding slightly later than babies receiving intermittent feeding, the evidence is of low certainty. However, the clinical risks and benefits of continuous and intermittent nasogastric tube milk feeding cannot be reliably discerned from current available randomised trials. Further research is needed to determine if either feeding method is more appropriate for the initiation of feeds. A rigorous methodology should be adopted, defining feeding protocols and feeding intolerance consistently for all infants. Infants should be stratified according to birth weight and gestation, and possibly according to illness.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
SSP declares that Hamilton Health Sciences Foundation funded the systematic review.
LC retired as a Registered Dietitian in the neonatal intensive care unit (NICU) at McMaster Children's Hospital in July 2020.
FS has no interest to declare.
Core editorial and administrative support for this review has been provided by a grant from The Gerber Foundation. The Gerber Foundation is a separately endowed, private foundation, independent from the Gerber Products Company. The grantor has no input on the content of the review or the editorial process (Sources of support).
Figures
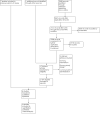
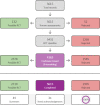

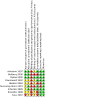

















Update of
-
Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams.Cochrane Database Syst Rev. 2011 Nov 9;2011(11):CD001819. doi: 10.1002/14651858.CD001819.pub2. Cochrane Database Syst Rev. 2011. Update in: Cochrane Database Syst Rev. 2021 Jun 24;6:CD001819. doi: 10.1002/14651858.CD001819.pub3. PMID: 22071802 Free PMC article. Updated.
References
References to studies included in this review
Akintorin 1997 {published data only}
Dollberg 2000 {published and unpublished data}
Dsilna 2005 {published and unpublished data}
Macdonald 1992 {published and unpublished data}
-
- Macdonald PD, Skeoch CH, Carse H, Dryburgh F, Alroomi LG, Galea P, et al. Randomised trial of continuous nasogastric, bolus nasogastric, and transpyloric feeding in infants of birthweight under 1400 g. Archives of Disease in Childhood 1992;67(4 Spec No):429-31. [DOI: 10.1136/adc.67.4_spec_no.429] [PMID: ] - DOI - PMC - PubMed
Neelam 2018 {published data only}
-
- CTRI/2017/06/008792. Study to compare continuous feeding with bolus feeding in preterm infants less than 32 weeks and less than 1250 g. http://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=12803&... (first received 8 June 2017).
-
- Neelam K, Vijay K, Pankaj G, Anup T. Comparison of continuous versus intermittent bolus feeding in preterm infants ≤ 32 weeks and ≤ 1250 g. Journal of Pediatrics and Child Health 2018;54(Supplement 1):37-8.
-
- Thakur A, Kumar V, Kler N, Garg P, Modi M, Soni A, et al. Comparison of continuous versus intermittent bolus feeding in preterm infants ≤ 32 weeks and ≤ 1250 g. Journal of Pediatric Gastroenterology and Nutrition 2018;66(Supplement 2):1103.
Rövekamp‐Abels 2015 {published data only}
-
- NL871 (NTR885). The effect of intermittent bolus nasogastric milk feeding versus semi-continuous milk feeding in preterm infants on TOLerance. trialregister.nl/trial/871 (first received 8 February 2007).
-
- Rövekamp-Abels LW, Hogewind-Schoonenboom JE, Wijs-Meijler DP, Maduro MD, Jansen-van der Weide MC, Van Goudoever JB, et al. Intermittent bolus or semi-continuous feeding for preterm infants? A randomised controlled trial. Journal of Pediatric Gastroenterology and Nutrition 2015;61(6):659-64. [DOI: 10.1097/MPG.0000000000000888] [PMID: ] - DOI - PubMed
Schanler 1999 {published and unpublished data}
Silvestre 1996 {published data only}
Toce 1987 {published data only}
-
- Toce SS, Keenan WJ, Homan SM. Enteral feeding in very-low-birth-weight infants. A comparison of two nasogastric methods. American Journal of Diseases of Children 1987;141(4):439-44. [DOI: 10.1001/archpedi.1987.04460040097025] [PMID: ] - PubMed
References to studies excluded from this review
Baker 1997 {published data only}
Berseth 1992 {published data only}
-
- Berseth CL, Baker J, DeVille K, Jadcherla S. Rate of feeding and nutrient concentration affect preterm small intestinal motor responses to orogastric feedings. Gastroenterology 1992;103:1385. [DOI: 10.1016/0016-5085(92)91589-V] - DOI
Bozzetti 2012 {published data only}
-
- Bozzetti V, Paterlini G, Meroni V, DeLorenzo P, Gazzolo D, Van Bel F, et al. Evaluation of splanchnic oximetry, Doppler flow velocimetry in the superior mesenteric artery and feeding tolerance in very low birthweight IUGR and non-IUGR infants receiving bolus versus continuous enteral nutrition. BMC Pediatrics 2012;12:106. [DOI: 10.1186/1471-2431-12-106] [PMID: ] - DOI - PMC - PubMed
Bozzetti 2016 {published data only}
Cordero Gonzalez 2020 {published data only}
-
- Cordero González G, Valdés Vázquez NO, Izaguirre Alcántara DD, Michel Macías C, Carrera Muiños S, Morales Barquet DA, et al. Management of abdominal distension in the preterm infant with noninvasive ventilation: comparison of cenit versus 2x1 technique for the utilization of feeding tube. Journal of Neonatal-Perinatal Medicine 2020;13(3):367-72. [DOI: 10.3233/NPM-190301] [PMID: ] - DOI - PubMed
IRCT201408205168N7 {published data only}
-
- IRCT201408205168N7. The comparison early outcomes intermittent gavage in preterm infants: randomized clinical trial. en.irct.ir/trial/5520 (first received 15 March 2015).
Jajoo 2013 {published data only}
-
- Jajoo M, Arora N, Dabas V, Talukdar B, Kumari S. Early total versus gradual advancement of enteral nutrition in healthy preterm very low birth weight neonates in a tertiary care nursery: a randomised controlled trial. In: Pediatric Academic Societies Annual Meeting; 2013 May 4 -7; Washington (DC), USA. 2013.
Kempley 2014 {published data only}
-
- Kempley S, Gupta N, Linsell L, Dorling J, McCormick K, Mannix P, et al, ADEPT Trial Collaborative Group. Feeding infants below 29 weeks' gestation with abnormal antenatal Doppler: analysis from a randomised trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(1):F6-11. [DOI: 10.1136/archdischild-2013-304393] [PMID: ] - DOI - PubMed
Nangia 2015 {published data only}
-
- Nangia S, Vadivel V, Saili A, Thukral A. Comparison of early total enteral feeding (ETEF) vs. conventional enteral feeding (CEF) in stable very low birthweight (VLBW) infants - a randomized control trial. In: Pediatric Academic Societies (PAS) Annual Meeting; 2015 April 25 - 28; San Diego (CA), USA. 2015.
NCT01341236 {published data only}
-
- NCT01341236. Near infrared spectroscopy (NIRS) and superior mesenteric artery (SMA) Doppler patterns as predictor of feeding tolerance in very low birth weight (VLBW) intra uterine growth restricted (IUGR) and non IUGR Infants. clinicaltrials.gov/ct2/show/NCT01341236 (first received 25 April 2011).
NCT02915549 {published data only}
-
- NCT02915549. Early progressive feeding in human-milk fed extremely preterm infants: a randomized trial. clinicaltrials.gov/ct2/show/NCT02915549 (first received 27 February 2016).
Ng 2016 {published data only}
Richmond 2017 {published data only}
-
- Richmond C, Birch P. Feeding interventions because of respiratory events in preterm infants a randomised triple crossover study. Journal of Paediatrics and Child Health. Special Issue: Abstracts for the RACP Congress 2017. Bringing Specialists Together. Sharing Knowledge. Building Skills. 8–10 May 2017, Melbourne Convention and Exhibition Centre 2017;53(S3):4-5. [DOI: 10.1111/jpc.13572_4] - DOI
Sokou 2019 {published data only}
-
- Sokou R, Grivea G, Konstantinidi A, Antonogeorgos G, Kokori F, Varhalama E, et al. Feeding mode and gastric emptying time in neonates with gestational age. Journal of Perinatal Medicine 2019;47:eA298. [CENTRAL: CN-02074445] [DOI: 10.1515/jpm-2019-2501] [EMBASE: 630689439] - DOI
Xu 2014 {published data only}
-
- Xu YM, Zhu XP, Xiao Z, Yu L, Zhao X25551971. Influence of aggressive nutritional support on growth and development of very low birthweight infants. Clinical and Experimental Obstetrics & Gynecology 2014;41(6):717-22. [PMID: ] - PubMed
References to studies awaiting assessment
Corbin 2011 {published data only}
-
- Corbin LL, Smith S. Bolus versus longer infusion of enteral feedings for preterm neonates. Journal of Investigative Medicine 2011;59(1):168-9. [CENTRAL: CN-01738982] [DOI: 10.231/JIM.0b013e31820501bd] [EMBASE: 70524586] - DOI
Additional references
Aynsley‐Green 1982
-
- Aynsley-Green A, Adrian TE, Bloom SR. Feeding and the development of enteroinsular hormone secretion in the preterm infant: effects of continuous gastric infusions of human milk compared with intermittent boluses. Acta Paediatrica Scandinavica 1982;71(3):379-83. [DOI: 10.1111/j.1651-2227.1982.tb09438.x] [PMID: ] - DOI - PubMed
Aynsley‐Green 1989
Aynsley‐Green 1990
Bertoncelli 2012
Corvaglia 2014
-
- Corvaglia L, Martini S, Aceti A, Capretti MG, Galletti S, Faldella G. Cardiorespirtory events with bolus versus continuous enteral feeding in healthy preterm infants. Journal of Pediatrics 2014;165:1255-7. - PubMed
Davidson 2020
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed January 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grant 1991
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA, on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2020
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Krishnan 1981
-
- Krishnan V, Satish M. Continuous (C) vs. intermittent (I) nasogastric (N/G) feeding in very low birthweight (VLBW) infants. Pediatric Research 1981;15:537. [DOI: 10.1203/00006450-198104001-00596] - DOI
Li 2014
Lucas 1986
Marshall 2018
Newell 1988
Noel‐Storr 2020
-
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane centralised search service showed high sensitivity identifying randomised controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [DOI: 10.1016/j.jclinepi.2020.08.008] [PMID: ] - DOI - PubMed
Noel‐Storr 2021
-
- Noel-Storr AH, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomised trials. Journal of Clinical Epidemiology 2021;S0895-4356(21):00008-1. [DOI: 10.1016/j.jclinepi.2021.01.006] [PMID: 33476769] - DOI - PubMed
Ovelman 2020
-
- Ovelman C, Eckert C, Friesen C. Validating Cochrane Neonatal’s standard search databases: is it okay to stop searching Embase? In: Advances in Evidence Synthesis: special issue. Cochrane Database of Systematic Reviews 2020; (9 Suppl 1): [320]. Available from: cochranelibrary.com/cdsr/doi/10.1002/14651858.CD202001/full.
Raudonis 1995
-
- Raudonis BM, Talbot LA. Use of critical thinking in research: the research critique. In: Talbot LA, editors(s). Principles and Practice of Nursing Research. St. Louis (MO): Mosby, 1995:513-34.
Review Manager 2020 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rogers 2010
Rysavy 2020
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Thomas 2020
-
- Thomas J, McDonald S, Noel-Storr A, Shemilt I, Elliott J, Mavergames C, et al. Machine learning reduces workload with minimal risk of missing studies: development and evaluation of an RCT classifier for Cochrane Reviews. Journal of Clinical Epidemiology 2020;S0895-4356(20):31172-0. [DOI: 10.1016/j.jclinepi.2020.11.003] [PMID: ] - DOI - PMC - PubMed
Urrutia 1983
-
- Urrutia J, Poole E. Continuous nasogastric versus intermittent gavage feedings in very low birthweight infants. Pediatric Research 1983;17:203A.
Valman 1972
Wan 2014
Wang 2020
References to other published versions of this review
Premji 1999
Premji 2001
Premji 2003
Premji 2004
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

