A Versatile Silver(I) Pentafluorooxosulfate Reagent for the Synthesis of OSF5 Compounds
- PMID: 34165887
- PMCID: PMC8456853
- DOI: 10.1002/anie.202107587
A Versatile Silver(I) Pentafluorooxosulfate Reagent for the Synthesis of OSF5 Compounds
Abstract
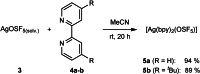
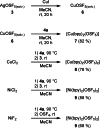
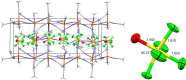
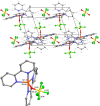
Herein, we report the preparation of silver(I) pentafluorooxosulfate from commercially available AgF and OSF4 . The compound is surprisingly stable in a MeCN solution. Apart from that, AgOSF5 has been stabilised by the addition of 2,2'-bipyridine ligands. Starting from solutions of the unstabilised silver(I) salt, OSF5 complexes with NiII , CuI , and CuII -centres have been obtained. In addition, AgOSF5 has proven to be generally capable of mediating the transfer of OSF5 groups to aryne moieties, thus furnishing a new and safe method for the preparation of OSF5 -substituted arenes. X-ray crystal structure analysis of selected transition-metal OSF5 compounds have revealed distorted octahedral [OSF5 ]- anions which are extensively stabilised by hydrogen bonding.
Keywords: arynes; chalcogens; fluorine; silver; synthetic methods.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
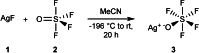
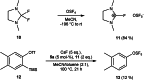
References
-
- For a review on OEF5 compounds, see: Seppelt K., Angew. Chem. Int. Ed. Engl. 1982, 21, 877–888;
- Angew. Chem. 1982, 94, 890–901.
-
- None
-
- Seppelt K., Nothe D., Inorg. Chem. 1973, 12, 2727–2730;
-
- Engelbrecht A., Sladky F., Inorg. Nucl. Chem. Lett. 1965, 1, 15;
-
- Mayer E., Sladky F., Inorg. Chem. 1975, 14, 589–592.
Publication types
LinkOut - more resources
Full Text Sources

