Functionalized Mouth-Conformable Interfaces for pH Evaluation of the Oral Cavity
- PMID: 34165900
- PMCID: PMC8224410
- DOI: 10.1002/advs.202003416
Functionalized Mouth-Conformable Interfaces for pH Evaluation of the Oral Cavity
Abstract
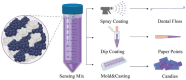
Oral health monitoring is highly desired, especially for in home use, however, current methods are not sensitive enough and technically convoluted for this purpose. This paper presents incorporation of bioactive materials and colorimetric chemical sensors into routinely used oral appliances transforming them into bioresponsive, conformable interfaces. Specifically, endodontic paper points and dental floss can be functionalized to locally sense and monitor pH variations within the oral cavity via color changes. Moreover, edible colorimetric indicators are developed and used to make sensing, edible devices in the form factor of candies that can dynamically and visually respond to acidity changes in saliva. These interfaces would enable early detection of caries (e.g., using dental floss and paper points) providing low-cost point of care devices that respond in real-time by detecting pH variations in biological fluids thus bringing monitoring to home settings .
Keywords: colorimetric sensors; mouth compatible interfaces; oral cavity.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
Tufts University has filed a patent based on this work and will seek to commercialize this technology.
Figures

References
-
- a) Chojnowska S., Baran T., Wilińska I., Sienicka P., Cabaj‐Wiater I., Knaś M., Adv. Med. Sci. 2018, 63, 185; - PubMed
- b) Henson B. S., Wong D. T., in Oral Biology: Molecular Techniques and Applications, (Eds: Seymour G. J., Cullinan M. P., Heng N. C. K.), Humana Press, Totowa, NJ: 2010, p. 21;
- c) Devi T. J., IOSR J. Dent. Med. Sci. 2014, 13, 52;
- d) Meagher R., Kousvelari E., Oral Dis. 2018, 24, 194; - PubMed
- e) Giannobile W. V., J. Am. Dent. Assoc. 2012, 143, 6S. - PubMed
-
- a) Bellagambi F. G., Lomonaco T., Salvo P., Vivaldi F., Hangouët M., Ghimenti S., Biagini D., Di Francesco F., Fuoco R., Errachid A., TrAC, Trends Anal. Chem. 2019, 124, 115781;
- b) Bhattarai K. R., Kim H.‐R., Chae H.‐J., Int. J. Med. Sci. 2018, 15, 823; - PMC - PubMed
- c) Yamuna P., Muthu P., Int. J. Oral Health Dent. 2017, 3, 149.
-
- a) Heikenfeld J., Jajack A., Feldman B., Granger S. W., Gaitonde S., Begtrup G., Katchman B. A., Nat. Biotechnol. 2019, 37, 407; - PubMed
- b) Gug I. T., Tertis M., Hosu O., Cristea C., TrAC, Trends Anal. Chem. 2019, 113, 301.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
