Genomic Classification and Clinical Outcome in Rhabdomyosarcoma: A Report From an International Consortium
- PMID: 34166060
- PMCID: PMC8425837
- DOI: 10.1200/JCO.20.03060
Genomic Classification and Clinical Outcome in Rhabdomyosarcoma: A Report From an International Consortium
Abstract
Purpose: Rhabdomyosarcoma is the most common soft tissue sarcoma of childhood. Despite aggressive therapy, the 5-year survival rate for patients with metastatic or recurrent disease remains poor, and beyond PAX-FOXO1 fusion status, no genomic markers are available for risk stratification. We present an international consortium study designed to determine the incidence of driver mutations and their association with clinical outcome.
Patients and methods: Tumor samples collected from patients enrolled on Children's Oncology Group trials (1998-2017) and UK patients enrolled on malignant mesenchymal tumor and RMS2005 (1995-2016) trials were subjected to custom-capture sequencing. Mutations, indels, gene deletions, and amplifications were identified, and survival analysis was performed.
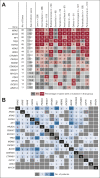
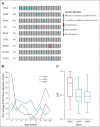
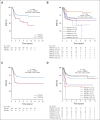
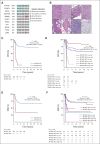
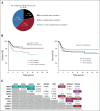
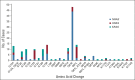
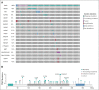
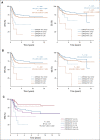
Results: DNA from 641 patients was suitable for analyses. A median of one mutation was found per tumor. In FOXO1 fusion-negative cases, mutation of any RAS pathway member was found in > 50% of cases, and 21% had no putative driver mutation identified. BCOR (15%), NF1 (15%), and TP53 (13%) mutations were found at a higher incidence than previously reported and TP53 mutations were associated with worse outcomes in both fusion-negative and FOXO1 fusion-positive cases. Interestingly, mutations in RAS isoforms predominated in infants < 1 year (64% of cases). Mutation of MYOD1 was associated with histologic patterns beyond those previously described, older age, head and neck primary site, and a dismal survival. Finally, we provide a searchable companion database (ClinOmics), containing all genomic variants, and clinical annotation including survival data.
Conclusion: This is the largest genomic characterization of clinically annotated rhabdomyosarcoma tumors to date and provides prognostic genetic features that refine risk stratification and will be incorporated into prospective trials.
Conflict of interest statement
Figures



Comment in
-
A Step Forward in Realizing the Promise of Genomic Medicine for Childhood Rhabdomyosarcoma.J Clin Oncol. 2021 Sep 10;39(26):2851-2854. doi: 10.1200/JCO.21.01296. Epub 2021 Jun 28. J Clin Oncol. 2021. PMID: 34181486 Free PMC article. No abstract available.
-
[Genomic classification and survival of patients with rhabdomyosarcoma: a report from an international consortium].Strahlenther Onkol. 2022 Apr;198(4):404-407. doi: 10.1007/s00066-022-01908-3. Epub 2022 Mar 1. Strahlenther Onkol. 2022. PMID: 35230459 German. No abstract available.
References
-
- Missiaglia E Williamson D Chisholm J, et al. : PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J Clin Oncol 30:1670-1677, 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

