SARS-CoV-2 Serologic Assays Dependent on Dual-Antigen Binding Demonstrate Diverging Kinetics Relative to Other Antibody Detection Methods
- PMID: 34166066
- PMCID: PMC8373029
- DOI: 10.1128/JCM.01231-21
SARS-CoV-2 Serologic Assays Dependent on Dual-Antigen Binding Demonstrate Diverging Kinetics Relative to Other Antibody Detection Methods
Abstract
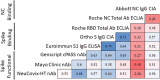
Longitudinal studies assessing durability of the anti-severe acute respiratory syndrome coronavirus 2 (anti-SARS-CoV-2) humoral immune response have generated conflicting results. This has been proposed to be due to differences in patient populations, the lack of standardized methodologies, and the use of assays that measure distinct aspects of the humoral response. SARS-CoV-2 antibodies were serially measured in sera from a cohort of 44 well-characterized convalescent plasma donors over 120 days post-COVID-19 symptom onset, utilizing eight assays, which varied according to antigen source, the detected antibody isotype, and the activity measured (i.e., binding, blocking, or neutralizing). While the majority of assays demonstrated a gradual decline in antibody titers over the course of 120 days, the two electrochemiluminescence immunoassay Roche assays (Roche Diagnostics Elecsys anti-SARS-CoV-2 [qualitative, nucleocapsid based] and Roche Diagnostics Elecsys anti-SARS-CoV-2 S [semiquantitative, spike based]), which utilize dual-antigen binding for antibody detection, demonstrated stable and/or increasing antibody titers over the study period. This study is among the first to assess longitudinal, rather than cross-sectional, SARS-CoV-2 antibody profiles among convalescent COVID-19 patients, primarily using commercially available serologic assays with Food and Drug Administration emergency use authorization. We show that SARS-CoV-2 antibody detection is dependent on the serologic method used, which has implications for future assay utilization and clinical value.
Keywords: COVID-19; SARS-CoV-2; antibodies; antibody; dual-antigen binding assays; immunoassays; serology.
Figures


References
-
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A, Nakao C, Rayaprolu V, Rawlings SA, Peters B, Krammer F, Simon V, Saphire EO, Smith DM, Weiskopf D, Sette A, Crotty S. 2021. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371:eabf4063. 10.1126/science.abf4063. - DOI - PMC - PubMed
-
- Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, Arnthorsson AO, Helgason D, Bjarnadottir K, Ingvarsson RF, Thorsteinsdottir B, Kristjansdottir S, Birgisdottir K, Kristinsdottir AM, Sigurdsson MI, Arnadottir GA, Ivarsdottir EV, Andresdottir M, Jonsson F, Agustsdottir AB, Berglund J, Eiriksdottir B, Fridriksdottir R, Gardarsdottir EE, Gottfredsson M, Gretarsdottir OS, Gudmundsdottir S, Gudmundsson KR, Gunnarsdottir TR, Gylfason A, Helgason A, Jensson BO, Jonasdottir A, Jonsson H, Kristjansson T, Kristinsson KG, Magnusdottir DN, Magnusson OT, Olafsdottir LB, Rognvaldsson S, Le Roux L, Sigmundsdottir G, Sigurdsson A, Sveinbjornsson G, Sveinsdottir KE, Sveinsdottir M, Thorarensen EA, Thorbjornsson B, Thordardottir M, Saemundsdottir J, et al.. 2020. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 383:1724–1734. 10.1056/NEJMoa2026116. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

