A variant of ASIC2 mediates sodium retention in nephrotic syndrome
- PMID: 34166227
- PMCID: PMC8410034
- DOI: 10.1172/jci.insight.148588
A variant of ASIC2 mediates sodium retention in nephrotic syndrome
Abstract
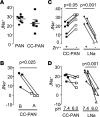
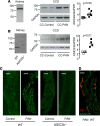
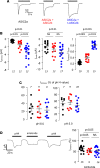
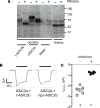
Idiopathic nephrotic syndrome (INS) is characterized by proteinuria and renal sodium retention leading to edema. This sodium retention is usually attributed to epithelial sodium channel (ENaC) activation after plasma aldosterone increase. However, most nephrotic patients show normal aldosterone levels. Using a corticosteroid-clamped (CC) rat model of INS (CC-PAN), we showed that the observed electrogenic and amiloride-sensitive Na retention could not be attributed to ENaC. We then identified a truncated variant of acid-sensing ion channel 2b (ASIC2b) that induced sustained acid-stimulated sodium currents when coexpressed with ASIC2a. Interestingly, CC-PAN nephrotic ASIC2b-null rats did not develop sodium retention. We finally showed that the expression of the truncated ASIC2b in the kidney was dependent on the presence of albumin in the tubule lumen and activation of ERK in renal cells. Finally, the presence of ASIC2 mRNA was also detected in kidney biopsies from patients with INS but not in any of the patients with other renal diseases. We have therefore identified a variant of ASIC2b responsible for the renal Na retention in the pathological context of INS.
Keywords: Homeostasis; Ion channels; Nephrology; Sodium channels.
Conflict of interest statement
Figures




Similar articles
-
Regulation of epithelial sodium channel in puromycin aminonucleoside-induced unilateral experimental nephrotic syndrome in normal and analbuminemic Nagase rats.Nephron Physiol. 2005;101(3):p51-62. doi: 10.1159/000086716. Epub 2005 Jul 12. Nephron Physiol. 2005. PMID: 16020936
-
Amiloride-insensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel.J Neurosci. 2003 May 1;23(9):3616-22. doi: 10.1523/JNEUROSCI.23-09-03616.2003. J Neurosci. 2003. PMID: 12736332 Free PMC article.
-
Nephrotic syndrome is associated with increased plasma K+ concentration, intestinal K+ losses, and attenuated urinary K+ excretion: a study in rats and humans.Am J Physiol Renal Physiol. 2019 Dec 1;317(6):F1549-F1562. doi: 10.1152/ajprenal.00179.2019. Epub 2019 Sep 30. Am J Physiol Renal Physiol. 2019. PMID: 31566427
-
Nephrotic syndrome: new concepts in the pathophysiology of sodium retention.J Nephrol. 2008 Nov-Dec;21(6):836-42. J Nephrol. 2008. PMID: 19034867 Review.
-
Mechanisms of renal NaCl retention in proteinuric disease.Acta Physiol (Oxf). 2013 Mar;207(3):536-45. doi: 10.1111/apha.12047. Epub 2013 Jan 7. Acta Physiol (Oxf). 2013. PMID: 23216619 Review.
Cited by
-
Nephrotic syndrome and adrenoleukodystrophy in a 5-year-old boy.Pediatr Nephrol. 2024 Dec;39(12):3463-3465. doi: 10.1007/s00467-024-06454-x. Epub 2024 Jul 11. Pediatr Nephrol. 2024. PMID: 38990330
-
Sodium retention in the nephrotic syndrome and the non-enzymatic function of prostasin.Pflugers Arch. 2022 Jun;474(6):567-568. doi: 10.1007/s00424-022-02684-w. Epub 2022 Mar 28. Pflugers Arch. 2022. PMID: 35344088 No abstract available.
-
Mice lacking γENaC palmitoylation sites maintain benzamil-sensitive Na+ transport despite reduced channel activity.JCI Insight. 2023 Nov 8;8(21):e172051. doi: 10.1172/jci.insight.172051. JCI Insight. 2023. PMID: 37707951 Free PMC article.
-
Role of ion channels in the mechanism of proteinuria (Review).Exp Ther Med. 2022 Nov 24;25(1):27. doi: 10.3892/etm.2022.11726. eCollection 2023 Jan. Exp Ther Med. 2022. PMID: 36561615 Free PMC article. Review.
-
Mechanistic insights into the primary and secondary alterations of renal ion and water transport in the distal nephron.J Intern Med. 2023 Jan;293(1):4-22. doi: 10.1111/joim.13552. Epub 2022 Aug 21. J Intern Med. 2023. PMID: 35909256 Free PMC article. Review.
References
-
- Frenk S, et al. Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc Soc Exp Biol Med. 1955;89(3):424–427. - PubMed
-
- Pedraza-Chaverri J, et al. Pathophysiology of experimental nephrotic syndrome induced by puromicyn aminonucleoside in rats. III. Effect of captopril, an angiotensin converting enzyme inhibitor, on proteinuria and sodium retention. Rev Invest Clin. 1990;42(3):210–216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

