COVID-19 Vaccination Coverage Among Adults - United States, December 14, 2020-May 22, 2021
- PMID: 34166331
- PMCID: PMC8224863
- DOI: 10.15585/mmwr.mm7025e1
COVID-19 Vaccination Coverage Among Adults - United States, December 14, 2020-May 22, 2021
Abstract
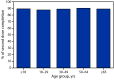
The U.S. COVID-19 vaccination program launched on December 14, 2020. The Advisory Committee on Immunization Practices recommended prioritizing COVID-19 vaccination for specific groups of the U.S. population who were at highest risk for COVID-19 hospitalization and death, including adults aged ≥75 years*; implementation varied by state, and eligibility was gradually expanded to persons aged ≥65 years beginning in January 2021. By April 19, 2021, eligibility was expanded to all adults aged ≥18 years nationwide.† To assess patterns of COVID-19 vaccination coverage among U.S. adults, CDC analyzed data submitted on vaccinations administered during December 14, 2020-May 22, 2021, by age, sex, and community-level characteristics. By May 22, 2021, 57.0% of persons aged ≥18 years had received ≥1 COVID-19 vaccine dose; coverage was highest among persons aged ≥65 years (80.0%) and lowest among persons aged 18-29 years (38.3%). During the week beginning February 7, 2021, vaccination initiation among adults aged ≥65 years peaked at 8.2%, whereas weekly initiation among other age groups peaked later and at lower levels. During April 19-May 22, 2021, the period following expanded eligibility to all adults, weekly initiation remained <4.0% and decreased for all age groups, including persons aged 18-29 years (3.6% to 1.9%) and 30-49 years (3.5% to 1.7%); based on the current rate of weekly initiation (as of May 22), younger persons will not reach the same levels of coverage as older persons by the end of August. Across all age groups, coverage (≥1 dose) was lower among men compared with women, except among adults aged ≥65 years, and lower among persons living in counties that were less urban, had higher social vulnerabilities, or had higher percentages of social determinants of poor health. Continued efforts to improve vaccination confidence and alleviate barriers to vaccination initiation, especially among adults aged 18-49 years, could improve vaccination coverage.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed.
Figures

References
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819–29. 10.1016/S0140-6736(21)00947-8 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

