Mycophenolate mofetil versus azathioprine in kidney transplant recipients on steroid-free, low-dose cyclosporine immunosuppression (ATHENA): A pragmatic randomized trial
- PMID: 34166370
- PMCID: PMC8224852
- DOI: 10.1371/journal.pmed.1003668
Mycophenolate mofetil versus azathioprine in kidney transplant recipients on steroid-free, low-dose cyclosporine immunosuppression (ATHENA): A pragmatic randomized trial
Abstract
Background: We compared protection of mycophenolate mofetil (MMF) and azathioprine (AZA) against acute cellular rejection (ACR) and chronic allograft nephropathy (CAN) in kidney transplant recipients on steroid-free, low-dose cyclosporine (CsA) microemulsion maintenance immunosuppression.
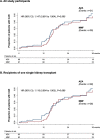
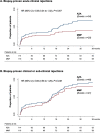
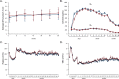
Methods and findings: ATHENA, a pragmatic, prospective, multicenter trial conducted by 6 Italian transplant centers, compared the outcomes of 233 consenting recipients of a first deceased donor kidney transplant induced with low-dose thymoglobulin and basiliximab and randomized to MMF (750 mg twice/day, n = 119) or AZA (75 to 125 mg/day, n = 114) added-on maintenance low-dose CsA microemulsion and 1-week steroid. In patients without acute clinical or subclinical rejections, CsA dose was progressively halved. Primary endpoint was biopsy-proven CAN. Analysis was by intention to treat. Participants were included between June 2007 and July 2012 and followed up to August 2016. Between-group donor and recipient characteristics, donor/recipient mismatches, and follow-up CsA blood levels were similar. During a median (interquartile range (IQR)) follow-up of 47.7 (44.2 to 48.9) months, 29 of 87 biopsied patients on MMF (33.3%) versus 31 of 88 on AZA (35.2%) developed CAN (hazard ratio (HR) [95% confidence interval (CI)]: 1.147 (0.691 to 1.904, p = 0.595). Twenty and 21 patients on MMF versus 34 and 14 on AZA had clinical [HR (95% CI): 0.58 (0.34 to 1.02); p = 0.057) or biopsy-proven subclinical [HR (95% CI): 1.49 (0.76 to 2.92); p = 0.249] ACR, respectively. Combined events [HR (95% CI): 0.85 (0.56 to 1.29); p = 0.438], patient and graft survival, delayed graft function (DGF), 3-year glomerular filtration rate (GFR) [53.8 (40.6;65.7) versus 49.8 (36.8;62.5) mL/min/1.73 m2, p = 0.50], and adverse events (AEs) were not significantly different between groups. Chronicity scores other than CAN predict long-term graft outcome. Study limitations include small sample size and unblinded design.
Conclusions: In this study, we found that in deceased donor kidney transplant recipients on low-dose CsA and no steroids, MMF had no significant benefits over AZA. This finding suggests that AZA, due to its lower costs, could safely replace MMF in combination with minimized immunosuppression.
Trial registration: ClinicalTrials.gov NCT00494741; EUDRACT 2006-005604-14.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- The Tricontinental Mycophenolate Mofetil Renal Transplantation Study Group. A blinded, randomized clinical trial of mycophenolate mofetil for the prevention of acute rejection in cadaveric renal transplantation. Transplantation. 1996;61:1029–37. - PubMed
-
- European Mycophenolate Mofetil Cooperative Study Group. Placebo-controlled study of mycophenolate mofetil combined with cyclosporin and corticosteroids for prevention of acute rejection. Lancet. 1995;345:1321–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

