CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma
- PMID: 34166502
- PMCID: PMC8893508
- DOI: 10.1182/blood.2020010543
CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma
Abstract
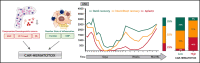
Hematotoxicity represents a frequent chimeric antigen receptor (CAR) T-cell-related adverse event and remains poorly understood. In this multicenter analysis, we studied patterns of hematopoietic reconstitution and evaluated potential predictive markers in 258 patients receiving axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) for relapsed/refractory large B-cell lymphoma. We observed profound (absolute neutrophil count [ANC] <100 cells per µL) neutropenia in 72% of patients and prolonged (21 days or longer) neutropenia in 64% of patients. The median duration of severe neutropenia (ANC < 500 cells per µL) was 9 days. We aimed to identify predictive biomarkers of hematotoxicity using the duration of severe neutropenia until day +60 as the primary end point. In the training cohort (n = 58), we observed a significant correlation with baseline thrombocytopenia (r = -0.43; P = .001) and hyperferritinemia (r = 0.54; P < .0001) on univariate and multivariate analysis. Incidence and severity of cytokine-release syndrome, immune effector cell-associated neurotoxicity syndrome, and peak cytokine levels were not associated with the primary end point. We created the CAR-HEMATOTOX model, which included markers associated with hematopoietic reserve (eg, platelet count, hemoglobin, and ANC) and baseline inflammation (eg, C-reactive protein and ferritin). This model was validated in independent cohorts, one from Europe (n = 91) and one from the United States (n = 109) and discriminated patients with severe neutropenia ≥14 days to <14 days (pooled validation: area under the curve, 0.89; sensitivity, 89%; specificity, 68%). A high CAR-HEMATOTOX score resulted in a longer duration of neutropenia (12 vs 5.5 days; P < .001) and a higher incidence of severe thrombocytopenia (87% vs 34%; P < .001) and anemia (96% vs 40%; P < .001). The score implicates bone marrow reserve and inflammation prior to CAR T-cell therapy as key features associated with delayed cytopenia and will be useful for risk-adapted management of hematotoxicity.
© 2021 by The American Society of Hematology.
Figures






Comment in
-
CAR T-cell hematotoxicity: is inflammation the key?Blood. 2021 Dec 16;138(24):2447-2448. doi: 10.1182/blood.2021012876. Blood. 2021. PMID: 34914831 No abstract available.
References
-
- Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

