Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7
- PMID: 34166617
- PMCID: PMC8185188
- DOI: 10.1016/j.celrep.2021.109292
Recurrent emergence of SARS-CoV-2 spike deletion H69/V70 and its role in the Alpha variant B.1.1.7
Abstract
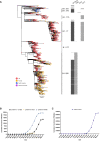
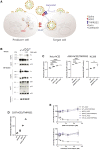
We report severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike ΔH69/V70 in multiple independent lineages, often occurring after acquisition of receptor binding motif replacements such as N439K and Y453F, known to increase binding affinity to the ACE2 receptor and confer antibody escape. In vitro, we show that, although ΔH69/V70 itself is not an antibody evasion mechanism, it increases infectivity associated with enhanced incorporation of cleaved spike into virions. ΔH69/V70 is able to partially rescue infectivity of spike proteins that have acquired N439K and Y453F escape mutations by increased spike incorporation. In addition, replacement of the H69 and V70 residues in the Alpha variant B.1.1.7 spike (where ΔH69/V70 occurs naturally) impairs spike incorporation and entry efficiency of the B.1.1.7 spike pseudotyped virus. Alpha variant B.1.1.7 spike mediates faster kinetics of cell-cell fusion than wild-type Wuhan-1 D614G, dependent on ΔH69/V70. Therefore, as ΔH69/V70 compensates for immune escape mutations that impair infectivity, continued surveillance for deletions with functional effects is warranted.
Keywords: Alpha variant; B.1.1.7; COVID-19; SARS-CoV-2; antibody escape; deletion; infectivity; neutralizing antibodies; resistance; spike mutation.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.D.M., C.S., K.C., E.C., L.P., and D.A.C. are employees of Vir Biotechnology and may hold shares in Vir Biotechnology. R.K.G. has received consulting fees from UMOVIS lab, Gilead Sciences, and ViiV Healthcare and a research grant from InvisiSmart Technologies.
Figures






References
-
- Bazykin G., Stanevich O., Danilenko D., Fadeev A., Komissarova K., Ivanova A., Sergeeva M., Safina K., Nabieva E., Klink G. 2021. Emergence of Y453F and δ69-70HV mutations in a lymphoma patient with long-term COVID-19.https://virological.org/t/emergence-of-y453f-and-69-70hv-mutations-in-a-...
Publication types
MeSH terms
Substances
Grants and funding
- MR/W005611/1/MRC_/Medical Research Council/United Kingdom
- 200594/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- BBS/E/I/COV07001/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MC_U105181010/MRC_/Medical Research Council/United Kingdom
- MC_UU_12014/12/MRC_/Medical Research Council/United Kingdom
- MR/R015600/1/MRC_/Medical Research Council/United Kingdom
- 204911/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_19026/MRC_/Medical Research Council/United Kingdom
- MR/P008801/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_19012/MRC_/Medical Research Council/United Kingdom
- MR/R024758/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_19027/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

