Recurrence of Acute Myocarditis Temporally Associated with Receipt of the mRNA Coronavirus Disease 2019 (COVID-19) Vaccine in a Male Adolescent
- PMID: 34166671
- PMCID: PMC8216855
- DOI: 10.1016/j.jpeds.2021.06.035
Recurrence of Acute Myocarditis Temporally Associated with Receipt of the mRNA Coronavirus Disease 2019 (COVID-19) Vaccine in a Male Adolescent
Figures
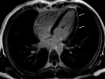
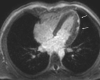
Comment in
-
Important Insights into Myopericarditis after the Pfizer mRNA COVID-19 Vaccination in Adolescents.J Pediatr. 2021 Nov;238:5. doi: 10.1016/j.jpeds.2021.07.057. Epub 2021 Jul 29. J Pediatr. 2021. PMID: 34332972 Free PMC article. No abstract available.
References
-
- US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in adolescents in another important action in fight against pandemic. FDA news release. May 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
-
- García JB, Ortega PP, Antonio Bonilla Fernández J, León AC, Burgos LR, Dorta EC. Miocarditis aguda tras administración de vacuna BNT162b2 contra la COVID-19 [Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19]. Rev Esp Cardiol [in Spanish]. doi: 10.1016/j.recesp.2021.03.009 - PMC - PubMed
-
- Centers for Disease Control and Prevention COVID-19 VaST Work Group technical report—May 17, 2021. Advisory Committee on Immunization Practices (ACIP) May 2021. https://www.cdc.gov/vaccines/acip/work-groups-vast/technical-report-2021...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

