Novel mutual prodrug of 5-fluorouracil and heme oxygenase-1 inhibitor (5-FU/HO-1 hybrid): design and preliminary in vitro evaluation
- PMID: 34167427
- PMCID: PMC8231349
- DOI: 10.1080/14756366.2021.1928111
Novel mutual prodrug of 5-fluorouracil and heme oxygenase-1 inhibitor (5-FU/HO-1 hybrid): design and preliminary in vitro evaluation
Abstract
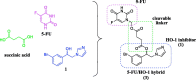
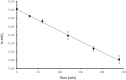
In this work, the first mutual prodrug of 5-fluorouracil and heme oxygenase1 inhibitor (5-FU/HO-1 hybrid) has been designed, synthesised, and evaluated for its in vitro chemical and enzymatic hydrolysis stability. Predicted in silico physicochemical properties of the newly synthesised hybrid (3) demonstrated a drug-like profile with suitable Absorption, Distribution, Metabolism, and Excretion (ADME) properties and low toxic liabilities. Preliminary cytotoxicity evaluation towards human prostate (DU145) and lung (A549) cancer cell lines demonstrated that 3 exerted a similar effect on cell viability to that produced by the reference drug 5-FU. Among the two tested cancer cell lines, the A549 cells were more susceptible for 3. Of note, hybrid 3 also had a significantly lower cytotoxic effect on healthy human lung epithelial cells (BEAS-2B) than 5-FU. Altogether our results served as an initial proof-of-concept to develop 5-FU/HO-1 mutual prodrugs as potential novel anticancer agents.
Keywords: 5-fluorouracil; HO-1 inhibitors; Mutual prodrugs; anticancer agents; heme oxygenase 1; hybrid compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
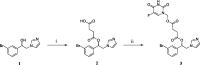



References
-
- Longley DB, Harkin DP, Johnston PG.. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer 2003;3:330–8. - PubMed
-
- Rich TA, Shepard RC, Mosley ST.. Four decades of continuing innovation with fluorouracil: Current and future approaches to fluorouracil chemoradiation therapy. J Clin Oncol 2004;22:2214–32. - PubMed
-
- Tanaka F, Fukuse T, Wada H, Fukushima M.. The history, mechanism and clinical use of oral 5-fluorouracil derivative chemotherapeutic agents. Curr Pharm Biotechnol 2000;1:137–64. - PubMed
-
- Manogue C, Cotogno P, Moses MM, et al. . Continuous infusion 5-fluorouracil (5fu) as a novel treatment for heavily pretreated prostate cancer patients: an update. J Clin Oncol 2019;37:319.
-
- Ibrahim T, Di Paolo A, Amatori F, et al. . Time-dependent pharmacokinetics of 5-fluorouracil and association with treatment tolerability in the adjuvant setting of colorectal cancer. J Clin Pharmacol 2012;52:361–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
