Randomized Trial Comparing Standard Versus Ultrasound-Assisted Thrombolysis for Submassive Pulmonary Embolism: The SUNSET sPE Trial
- PMID: 34167677
- PMCID: PMC9057455
- DOI: 10.1016/j.jcin.2021.04.049
Randomized Trial Comparing Standard Versus Ultrasound-Assisted Thrombolysis for Submassive Pulmonary Embolism: The SUNSET sPE Trial
Erratum in
-
Correction.JACC Cardiovasc Interv. 2021 Oct 11;14(19):2194. doi: 10.1016/j.jcin.2021.07.010. JACC Cardiovasc Interv. 2021. PMID: 34620402 No abstract available.
Abstract
Objectives: The aim of this trial was to determine whether ultrasound-assisted thrombolysis (USAT) is superior to standard catheter-directed thrombolysis (SCDT) in pulmonary arterial thrombus reduction for patients with submassive pulmonary embolism (sPE).
Background: Catheter-directed therapy has been increasingly used in sPE and massive pulmonary embolism as a decompensation prevention and potentially lifesaving procedure. It is unproved whether USAT is superior to SCDT using traditional multiple-side-hole catheters in the treatment of patients with pulmonary embolism.
Methods: Adults with sPE were enrolled. Participants were randomized 1:1 to USAT or SCDT. The primary outcome was 48-hour clearance of pulmonary thrombus assessed by pre- and postprocedural computed tomographic angiography using a refined Miller score. Secondary outcomes included improvement in right ventricular-to-left ventricular ratio, intensive care unit and hospital stay, bleeding, and adverse events up to 90 days.
Results: Eighty-one patients with acute sPE were randomized and were available for analysis. The mean total dose of alteplase for USAT was 19 ± 7 mg and for SCDT was 18 ± 7 mg (P = 0.53), infused over 14 ± 6 and 14 ± 5 hours, respectively (P = 0.99). In the USAT group, the mean raw pulmonary arterial thrombus score was reduced from 31 ± 4 at baseline to 22 ± 7 (P < 0.001). In the SCDT group, the score was reduced from 33 ± 4 to 23 ± 7 (P < 0.001). There was no significant difference in mean thrombus score reduction between the 2 groups (P = 0.76). The mean reduction in right ventricular/left ventricular ratio from baseline (1.54 ± 0.30 for USAT, 1.69 ± 0.44 for SCDT) to 48 hours was 0.37 ± 0.34 in the USAT group and 0.59 ± 0.42 in the SCDT group (P = 0.01). Major bleeding (1 stroke and 1 vaginal bleed requiring transfusion) occurred in 2 patients, both in the USAT group.
Conclusions: In the SUNSET sPE (Standard vs. Ultrasound-Assisted Catheter Thrombolysis for Submassive Pulmonary Embolism) trial, patients undergoing USAT had similar pulmonary arterial thrombus reduction compared with those undergoing SCDT, using comparable mean lytic doses and durations of lysis.
Keywords: EKOS; catheter thrombolysis; pulmonary embolism; pulmonary hypertension; ultrasound-assisted thrombolysis.
Copyright © 2021 American College of Cardiology Foundation. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures Dr. Avgerinos is a member of the Speakers Bureau for Boston Scientific; and is a consultant for AngioDynamics and BD Medical. Dr. Chaer is a member of the Speakers Bureau for Boston Scientific. Dr. Jaber is a consultant for Inari. Dr. Ross is a member of the Peripheral Intervention Vascular Senior Medical Council for Boston Scientific Corporation. Dr. Rivera-Lebron is a consultant for Bristol Myers Squibb. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures
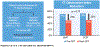
Comment in
-
Is it Time to Sunset Ultrasound-Assisted Catheter-Directed Thrombolysis for Submassive PE?JACC Cardiovasc Interv. 2021 Jun 28;14(12):1374-1375. doi: 10.1016/j.jcin.2021.05.020. JACC Cardiovasc Interv. 2021. PMID: 34167678 No abstract available.
References
-
- Giri J, Sista AK, Weinberg I, et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation 2019;140(20): e774–801. - PubMed
-
- Gayou EL, Makary MS, Hughes DR, et al. Nationwide trends in use of catheter-directed therapy for treatment of pulmonary embolism in Medicare beneficiaries from 2004 to 2016. J Vasc Interv Radiol 2019;30(6):801–6. - PubMed
-
- Stein PD, Matta F, Hughes MJ. Thrombolysis in submassive pulmonary embolism and acute cor pulmonale. Am J Cardiol 2020;131:109–14. - PubMed
-
- Chatterjee S, Chakraborty A, Weinberg I, et al. Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. JAMA 2014; 311(23):2414–21. - PubMed
-
- Avgerinos ED, Saadeddin Z, Abou Ali AN, et al. A meta-analysis of outcomes of catheter-directed thrombolysis for high- and intermediate-risk pulmonary embolism. J Vasc Surg Venous Lymphat Disord 2018;6(4):530–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

