Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma
- PMID: 34168004
- PMCID: PMC8231064
- DOI: 10.1136/jitc-2020-002305
Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma
Abstract
Background: Neutrophils play a controversial role in tumor development. The function of programmed cell death-1 ligand (PD-L1+) neutrophils, however, may inhibit the cytotoxicity of anti-tumor immunity. In this study, we elucidate the stimulators of PD-L1+ neutrophils in tumor microenvironment (TME) and explore the optimal combination to enhance the effect of lenvatinib by inhibiting PD-L1+ neutrophils in hepatocellular carcinoma.
Methods: Neutrophil infiltration after lenvatinib treatment was examined with RNA sequencing and multicolor flow cytometry analysis in patient samples, subcutaneous and orthotopic mouse models. Neutrophils and T cells were isolated from peripheral blood and tumor tissues and purified with magnetic beads for cytotoxicity assay. Metabolites and cytokines were detected by a biochemical analyzer manufactured by Yellow Springs Instrument (YSI) and proteome profiler cytokines array. In vitro screening of pathway inhibitors was used to identify possible candidates that could reduce PD-L1+ neutrophil infiltration. Further in vivo assays were used for verification.
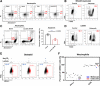
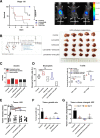
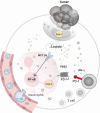
Results: Lenvatinib increased neutrophil recruitment by inducing CXCL2 and CXCL5 secretion in TME. After entering TME, neutrophils polarized toward N2 phenotype. PD-L1 expression was simultaneously upregulated. Thus, lenvatinib efficacy on tumor cells hindered. The increasing PD-L1+ neutrophils positively corelated with a suppressive T cell phenotype. Further investigation indicated that JAK/STAT1 pathway activated by immune-cell-derived interferon γ and MCT1/NF-kB/COX-2 pathway activated by high concentrations of tumor-derived lactate could induce PD-L1+ neutrophils. The latter could be significantly inhibited by COX-2 inhibitor celecoxib. Further in vivo assays verified that Celecoxib decreased the survival of lactate-stimulated PD-L1+ neutrophil and promoted the antitumor effect of lenvatinib.
Conclusions: PD-L1+ neutrophils decrease T cell cytotoxicity. Tumor-derived lactate induces PD-L1 expression on neutrophils via MCT1/NF-κB/COX-2 pathway. Thus, COX-2 inhibitor could reduce PD-L1+ neutrophil and restore T cell cytotoxicity. This may provide a potent addition to lenvatinib.
Keywords: combination; drug therapy; metabolic networks and pathways; neutrophil infiltration; programmed cell death 1 receptor; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
-
- Deng H, Kan A, Lyu N, et al. . Dual vascular endothelial growth factor receptor and fibroblast growth factor receptor inhibition elicits antitumor immunity and enhances programmed cell death-1 checkpoint blockade in hepatocellular carcinoma. Liver Cancer 2020;9:338–57. 10.1159/000505695 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
