Immune checkpoint inhibitor treatment and atherosclerotic cardiovascular disease: an emerging clinical problem
- PMID: 34168005
- PMCID: PMC8231062
- DOI: 10.1136/jitc-2021-002916
Immune checkpoint inhibitor treatment and atherosclerotic cardiovascular disease: an emerging clinical problem
Abstract
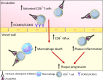
Antibody-mediated blockade of co-inhibitory molecules such as cytotoxic T lymphocyte-associated protein 4, PD1 and PDL1 elicits potent antitumor responses and improves the prognosis of many patients with cancer. As these immune checkpoint inhibitors (ICIs) are increasingly prescribed to a diverse patient population, a broad range of adverse effects is emerging. Atherosclerosis, a lipid-driven chronic inflammatory disease of the large arteries, may be aggravated by ICI treatment. In this review, we discuss recent clinical studies that analyze the correlation between ICI use and atherosclerotic cardiovascular disease (CVD). Indeed, several studies report an increased incidence of atherosclerotic CVD after ICI administration, with the occurrence of pathologies such as myocardial infarction, ischemic stroke and coronary artery disease significantly higher after ICI use. Increased awareness and better monitoring of ICI-treated patients can elucidate risk factors that contribute to ICI-induced aggravation of atherosclerosis and identify promising treatment strategies. For now, optimal cardiovascular risk assessment is required to protect ICI-receiving patients and long-term survivors of cancer from the detrimental effects of ICI therapy on atherosclerotic CVD.
Keywords: immunotherapy; inflammation; review.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
