Evaluation of Artesunate-mefloquine as a Novel Alternative Treatment for Schistosomiasis in African Children (SchistoSAM): protocol of a proof-of-concept, open-label, two-arm, individually-randomised controlled trial
- PMID: 34168029
- PMCID: PMC8231067
- DOI: 10.1136/bmjopen-2020-047147
Evaluation of Artesunate-mefloquine as a Novel Alternative Treatment for Schistosomiasis in African Children (SchistoSAM): protocol of a proof-of-concept, open-label, two-arm, individually-randomised controlled trial
Abstract
Introduction: Alternative drugs and diagnostics are needed for the treatment and control of schistosomiasis. The exclusive use of praziquantel (PZQ) in mass drug administration programmes may result in the emergence of drug resistance. PZQ has little activity against Schistosoma larvae, thus reinfection remains a problem in high-risk communities. Furthermore, the insufficient sensitivity of conventional microscopy hinders therapeutic response assessment. Evaluation of artesunate-mefloquine (AM) as a Novel Alternative Treatment for Schistosomiasis in African Children (SchistoSAM) aims to evaluate the safety and efficacy of the antimalarial combination artesunate-mefloquine, re-purposed for the treatment of schistosomiasis, and to assess the performance of highly sensitive novel antigen-based and DNA-based assays as tools for monitoring treatment response.
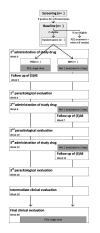
Methods and analysis: The SchistoSAM study is an open-label, two-arm, individually randomised controlled non-inferiority trial, with a follow-up of 48 weeks. Primary school-aged children from the Richard Toll district in northern Senegal, an area endemic for Schistosoma mansoni and Schistosoma haematobium, are allocated to the AM intervention arm (3-day courses at 6-week intervals) or the PZQ control arm (single dose of 40 mg/kg). The trial's primary endpoints are the efficacy (cure rate (CR), assessed by microscopy) and safety (frequency and pattern of drug-related adverse events) of one AM course versus PZQ at 4 weeks after treatment. Secondary endpoints include (1) cumulative CR, egg reduction rate and safety after each additional course of AM, and at weeks 24 and 48, (2) prevalence and severity of schistosomiasis-related morbidity and (3) malaria prevalence, incidence and morbidity, both after 24 and 48 weeks. CRs and intensity reduction rates are also assessed by antigen-based and DNA-based diagnostic assays, for which performance for treatment monitoring is evaluated.
Ethics and dissemination: Ethics approval was obtained both in Belgium and Senegal. Oral assent from the children and signed informed consent from their legal representatives was obtained, prior to enrolment. The results will be disseminated in peer-reviewed journals and at international conferences.
Trial registration number: NCT03893097; pre-results.
Keywords: adverse events; clinical trials; molecular diagnostics; parasitology; tropical medicine.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- WHO . Global health estimates 2016: deaths by cause, age, sex, by country and by region, 2000-2016, 2018.
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017;390:1211–59. 10.1016/S0140-6736(17)32154-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical