The Chronic Lymphocytic Leukemia Comorbidity Index (CLL-CI): A Three-Factor Comorbidity Model
- PMID: 34168050
- PMCID: PMC8416936
- DOI: 10.1158/1078-0432.CCR-20-3993
The Chronic Lymphocytic Leukemia Comorbidity Index (CLL-CI): A Three-Factor Comorbidity Model
Abstract
Purpose: Comorbid medical conditions define a subset of patients with chronic lymphocytic leukemia (CLL) with poor outcomes. However, which comorbidities are most predictive remains understudied.
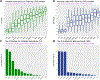
Experimental design: We conducted a retrospective analysis from 10 academic centers to ascertain the relative importance of comorbidities assessed by the cumulative illness rating scale (CIRS). The influence of specific comorbidities on event-free survival (EFS) was assessed in this derivation dataset using random survival forests to construct a CLL-specific comorbidity index (CLL-CI). Cox models were then fit to this dataset and to a single-center, independent validation dataset.
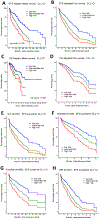
Results: The derivation and validation sets comprised 570 patients (59% receiving Bruton tyrosine kinase inhibitor, BTKi) and 167 patients (50% receiving BTKi), respectively. Of the 14 CIRS organ systems, three had a strong and stable influence on EFS: any vascular, moderate/severe endocrine, moderate/severe upper gastrointestinal comorbidity. These were combined to create the CLL-CI score, which was categorized into 3 risk groups. In the derivation dataset, the median EFS values were 58, 33, and 20 months in the low, intermediate, and high-risk groups, correspondingly. Two-year overall survival (OS) rates were 96%, 91%, and 82%. In the validation dataset, median EFS values were 81, 40, and 23 months (two-year OS rates 97%/92%/88%), correspondingly. Adjusting for prognostic factors, CLL-CI was significantly associated with EFS in patients treated with either chemo-immunotherapy or with BTKi in each of our 2 datasets.
Conclusions: The CLL-CI is a simplified, CLL-specific comorbidity index that can be easily applied in clinical practice and correlates with survival in CLL.
©2021 American Association for Cancer Research.
Figures

References
-
- Salvi F, Miller MD, Grilli A, Giorgi R, Towers AL, Morichi V, et al.A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc 2008;56(10):1926–31 doi JGS1935 [pii] 10.1111/j.1532-5415.2008.01935.x. - DOI - PubMed

